Abstract
Avian metapneumovirus (aMPV) causes an acute and highly contagious upper respiratory tract infection in turkeys and chickens. In this study, a competitive ELISA (C-ELISA) was developed for the detection of antibodies to aMPV in chicken sera and/or their egg yolks. This assay is based on the competitive binding of monoclonal antibody with serum antibodies to recombinant aMPV N protein expressed by a recombinant baculovirus. The C-ELISA showed specificity and sensitivity of 100% and 98.0%, respectively, when compared to the virus neutralization test. In specific pathogen-free chickens experimentally infected with aMPV SC1509 strain, the C-ELISA started to detect antibodies to aMPV as early as 5 days post infection from birds infected with aMPV, while a commercial ELISA kit detected first 10 days post infection. The C-ELISA was similar or superior to a commercial ELISA kit when serum and egg yolk samples collected from chickens on six outbreak farms were tested for diagnosis. The C-ELISA developed in the present work provides a short turnaround time and can be a useful diagnostic and screening tool for aMPV infection in the field.
Go to : 
REFERENCES
1). Pringle CR. Virus taxonomy–San Diego 1998. Arch Virol. 1998. 143:1449–59.
2). Ling R., Easton AJ., Pringle CR. Sequence analysis of the 22K, SH and G genes of turkey rhinotracheitis virus and their intergenic regions reveals a gene order different from that of other pneumoviruses. J Gen Virol. 1992. 73:1709–15.

3). Baxter-Jones C., Grant M., Jones RC., Wilding GP. A comparison of three methods for detecting antibodies to turkey rhinotracheitis virus. Avian Pathol. 1989. 18:91–8.
4). Jones RC. Avian pneumovirus infection: Questions still unanswered. Avian Pathol. 1996. 25:639–48.

5). Collins MS., Gough RE., Alexander DJ. Antigenic differentiation of avian pneumovirus isolates using polyclonal antisera and mouse monoclonal antibodies. Avian Pathol. 1993. 22:469–79.

6). Cook JKA., Jones BV., Ellis MM., Jing L., Cavanagh D. Antigenic differentiation of strains of turkey rhinotracheitis virus using monoclonal antibodies. Avian Pathol. 1993. 22:257–73.

7). Juhasz K., Easton AJ. Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: evidence for two distinct subgroups. J Gen Virol. 1994. 75:2873–80.

8). Aung YH., Liman M., Neumann U., Rautenschlein S. Reproducibility of swollen sinuses in broilers by experimental infection with avian metapneumovirus subtypes A and B of turkey origin and their comparative pathogenesis. Avian Pathol. 2008. 37:65–74.
10). Lwamba HC., Bennett RS., Lauer DC., Halvorson DA., Njenga MK. Characterization of avian metapneumoviruses isolated in the USA. Anim Health Res Rev. 2002. 3:107–17.

11). Bayon-Auboyer MH., Jestin V., Toquin D., Cherbonnel M., Eterradossi N. Comparison of F-, G- and N-based RT-PCR protocols with conventional virological procedures for the detection and typing of turkey rhinotracheitis virus. Arch Virol. 1999. 144:1091–109.
12). Toquin D., Bayon-Auboyer MH., Eterradossi N., Jestin V. Isolation of a pneumovirus from a Muscovy duck. Vet Rec. 1999. 145:680.
14). McDougall JS., Cook JKA. Turkey rhinotracheitis: preliminary investigations. Vet Rec. 1986. 118:206–7.

15). Picault JP., Giraud P., Drouin P., Guittet M., Bennejean G., Lamande J, et al. Isolation of a TRT-like virus from chickens with swollen head syndrome. Vet Rec. 1987. 121:135.
16). Shin HJ., Nagaraja KV., McComb B., Halvorson DA., Jirjis FF., Shaw DP, et al. Isolation of avian pneumovirus from mallard ducks that is genetically similar to viruses isolated from neighboring commercial turkeys. Virus Res. 2002. 83:207–12.

17). Catelli E., De Marco MA., Delogu M., Terregino C., Guberti V. Serological evidence of avian pneumovirus infection in reared and free-living pheasants. Vet Rec. 2001. 149:56–8.

18). Cadman HF., Kelly PJ., Zhou R., Davelaar F., Mason PR. A serosurvey using enzymelinked immunosorbent assay for antibodies against poultry pathogens in Ostriches (Struthio camelus) from Zimbabwe. Avian Dis. 1994. 38:621–5.
19). Gough RE., Collins MS., Cox WJ., Chettle NJ. Experimental infection of turkeys, chickens, ducks, geese, guinea fowl, pheasants and pigeons with turkey rhinotracheitis virus. Vet Rec. 1988. 123:58–9.

20). Bennett RS., McComb B., Shin HJ., Njenga MK., Nagaraja KV., Halvorson DA. Detection of avian pneumovirus in wild Canada (Branta canadensis) and blue-winged teal (Anas discors) geese. Avian Dis. 2002. 46:1025–9.
21). Shin HJ., Njenga MK., McComb B., Halvorson DA., Nagaraja KV. Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys. J Clin Microbiol. 2000. 38:4282–4.

22). Cook JKA., Cavanagh D. Detection and differentiation of avian pneumoviruses (metapneumoviruses). Avian Pathol. 2002. 31:117–32.

23). Kim JH., Song CS., Seong HW., Mo IP., Kwon JH., Kim KS, et al. The investigation of sero-prevalence and occurrence of SHS from broiler breeder in Korea. Korean J Vet Res. 1992. 32:25.
24). Kim JE., Hwang JY., Bae DR., Sung MS., Kim ST., Kim SY. Examination of seroprevalence and detection of avian pneumovirus from layer hens in Gyeongbuk province. Korean J Vet Serv. 2007. 30:43–9.
25). Kim ST., Kim SK., Cho MH., Kim YH. Serological survey of avian pneumovirus infection in laying hens of Gyeongbuk province. Korean J Vet Serv. 2003. 26:51–6.
26). Lee JW., Shon KR., Park KS., Kim YT., Kim CC., Han KS, et al. Serological survey of avian pneumovirus and reovirus in breeders of Jeonbuk province. Korean J Vet Serv. 2006. 29:9–18.
27). Park JB., Cha SY., Park YM., Zhao DD., Song HJ., Jang HK. Recently epidemiological survey of the viral diseases of broiler chickens in Jeonbuk province from 2005 to 2007. Korean J Vet Serv. 2008. 31:43–55.
28). Kwon JS., Lee HJ., Jeong SH., Park JY., Hong YH., Lee YJ, et al. Isolation and characterization of avian metapneumovirus from chickens in Korea. J Vet Sci. 2010. 11:59–66.

29). Choi KS., Jeon WJ., Park MJ., Lee EK., Kwon JH. Isolation and characterization of avian metapneumovirus from broiler breeder chickens in Korea. J Bacteriol Virol. 2009. 39:373–82.

30). Gulati BR., Cameron KT., Seal BS., Goyal SM., Halvorson DA., Njenga MK. Development of a highly sensitive and specific enzyme-linked immunosorbent assay based on recombinant matrix protein for detection of avian pneumovirus antibodies. J Clin Microbiol. 2000. 38:4010–4.

31). Gulati BR., Munir S., Patnayak DP., Goyal SM., Kapur V. Detection of antibodies to U.S. isolates of avian pneumovirus by a recombinant nucleocapsid protein-based sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 2001. 39:2967–70.

32). Hamelin ME., Boivin G. Development and validation of an enzyme-linked immunosorbent assay for human metapneumo-virus serology based on a recombinant viral protein. Clin Diagn Lab Immunol. 2005. 12:249–53.

33). Liu L., Qian Y., Zhu R., Zhao L., Deng J. Generation of recombinant nucleocapsid protein of human metapneumo-virus in baculovirus for detecting antibodies in the Beijing population. Arch Virol. 2010. 155:47–54.

34). Reed LJ., Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938. 27:493–7.
35). Lee YJ. Studies on detection of turkey rhinotracheitis virus using monoclonal antibody and polymerase chain reaction. Master's Thesis. Konkuk University. 1995.
36). Choi KS., Nah JJ., Ko YJ., Choi CU., Kim JH., Kang SY, et al. Characterization of antigenic sites on the rinderpest virus N protein using monoclonal antibodies. J Vet Sci. 2003. 4:57–65.
37). Choi KS., Nah JJ., Choi CU., Ko YJ., Sohn HJ., Libeau G, et al. Monoclonal antibody-based competitive ELISA for simultaneous detection of rinderpest virus and peste des petits ruminants virus antibodies. Vet Microbiol. 2003. 96:1–16.

38). Fooks AR., Stephenson JR., Warnes A., Dowsett BA., Rima BK., Wilkinson GW. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structure. J Gen Virol. 1993. 74:1439–44.
39). Ismail TM., Yamanaka MK., Saliki JT., El-Kholy A., Mebus C., Yilma T. Cloning and expression of the nucleoprotein of peste des petits ruminants virus in baculovirus for use in serological diagnosis. Virology. 1995. 208:776–8.

40). Buraphacheep W., Britt WJ., Sullender WM. Detection of antibodies to respiratory syncytial virus attachment and nucleocapsid proteins with recombinant baculovirus-expressed antigens. J Clin Microbiol. 1997. 35:354–7.

41). Li J., Ling R., Randhawa JS., Shaw K., Davis PJ., Juhasz K, et al. Sequence of the nucleocapsid protein gene of subgroup A and B avian pneumoviruses. Virus Res. 1996. 41:185–91.

Go to : 
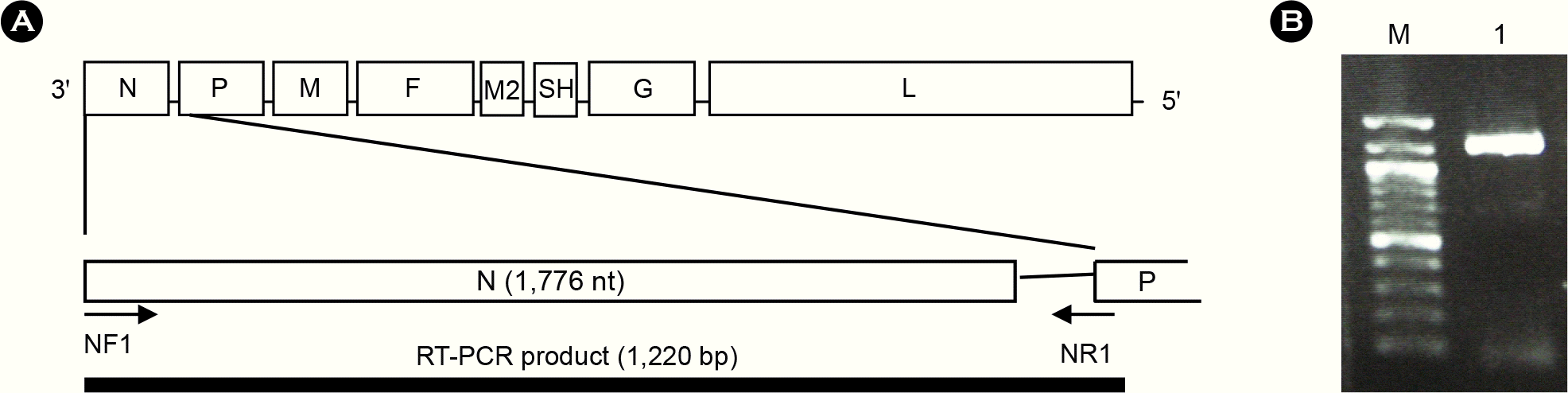 | Figure 1.RT-PCR amplification of full-length N gene of aMPV from a nasal turbinate sample of aMPV-infected chicken. (A) Schematic diagram for amplification of full-length N gene of aMPV by RT-PCR using primers NF1 and NR1. (B) RT-PCR result. M, molecular marker; Lane 1, nasal turninate sample. |
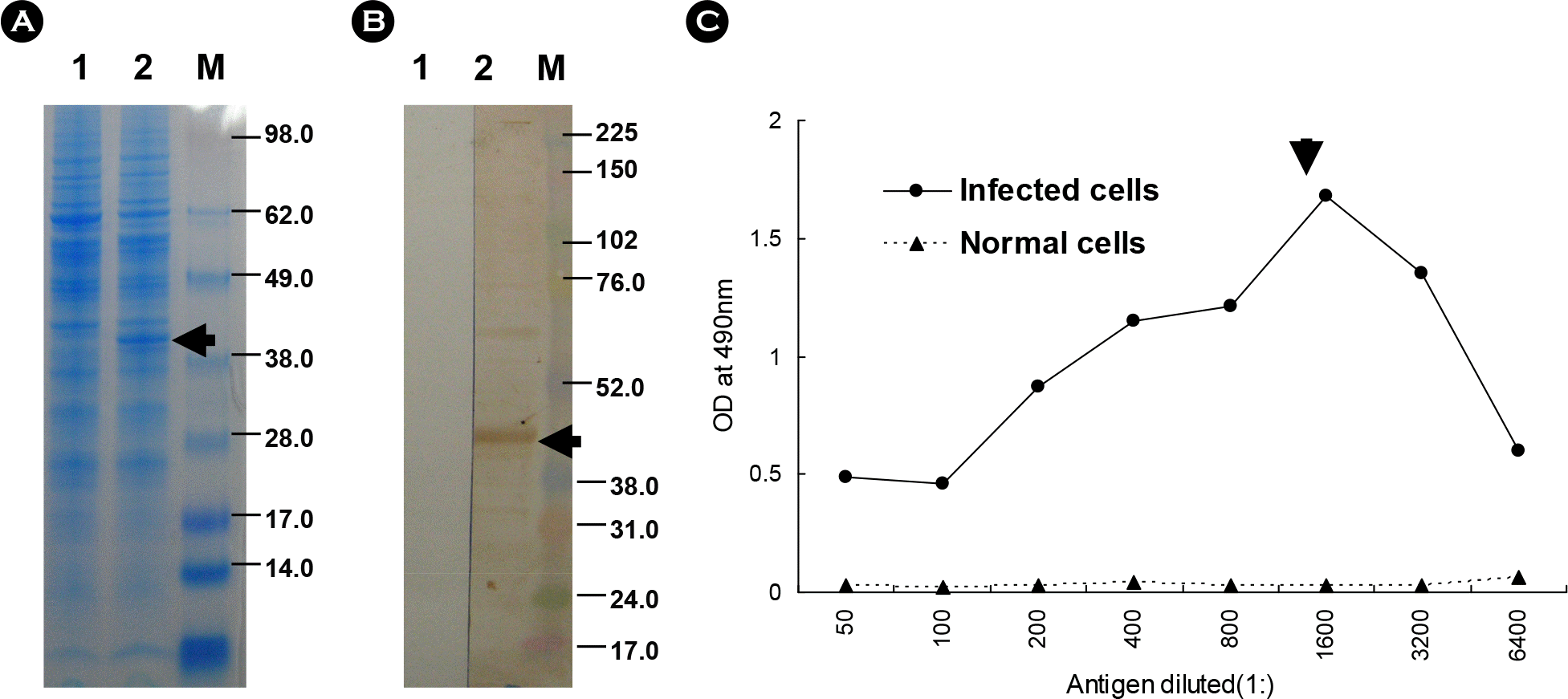 | Figure 2.Expression of recombinant aMPV N protein by the rBac/AMPVN. The cell extracts of expressed protein were either fractionated using SDS-PAGE and then visualized by Commassie blue staining (A) and immunoblotting using anti-aMPV antiserum (B) or titrated by ELISA using mAb12.2.1 (C). Lane 1, normal Sf9 cells; lane 2, Sf9 cells infected with rBac/AMPVN; M, molecular marker. Arrow represents recombinant protein having molecular weight of 44 kDa. |
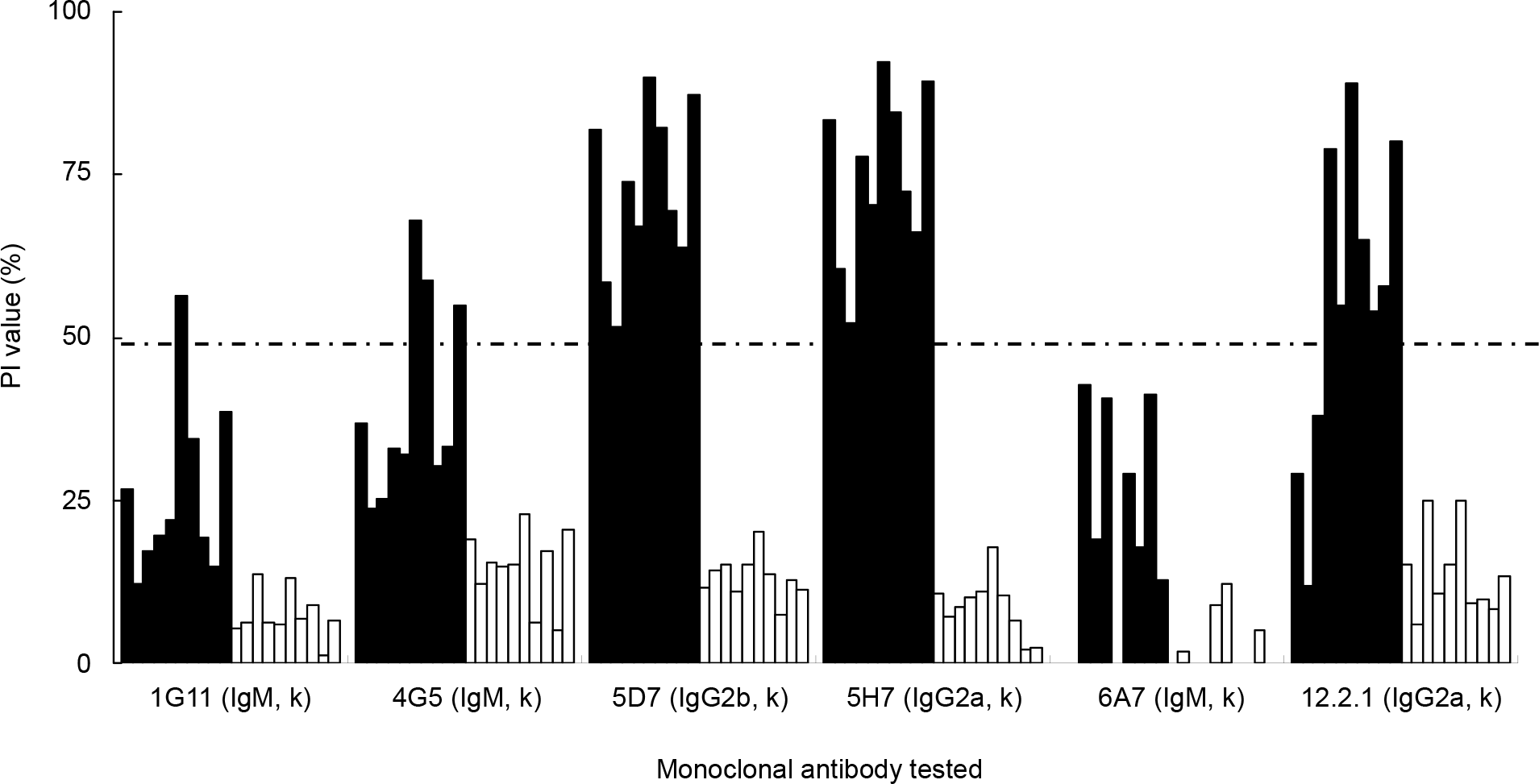 | Figure 3.Competitive binding of mAbs and serum antibodies to the recombinant aMPV N protein in a competitive ELISA. Serum panel comprising 10 aMPV antibody-positive and 10 aMPV antibody-negative chicken sera were used for the ELISA. Filled and empty bars represent positive and negative chicken sera, respectively. |
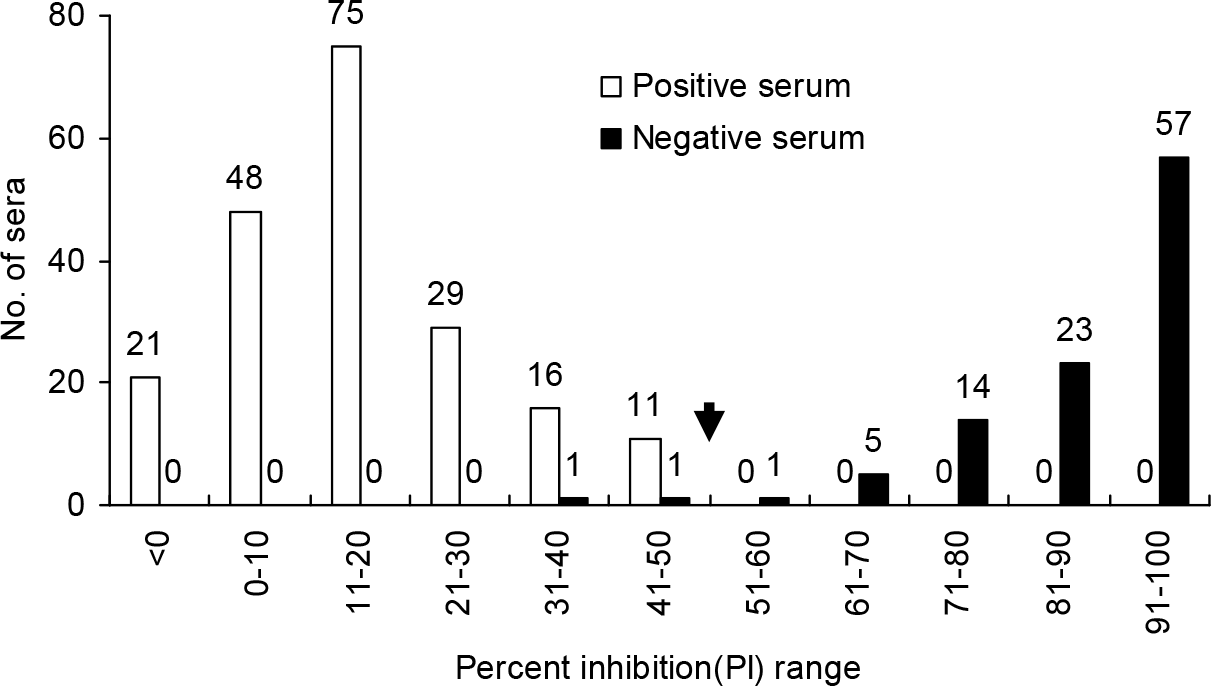 | Figure 4.Sensitivity and specificity of a competitive ELISA using positive (n=102) and negative (n = 200) chicken sera. Arrow indicates the cutoff value of 50 in the C-ELISA. |
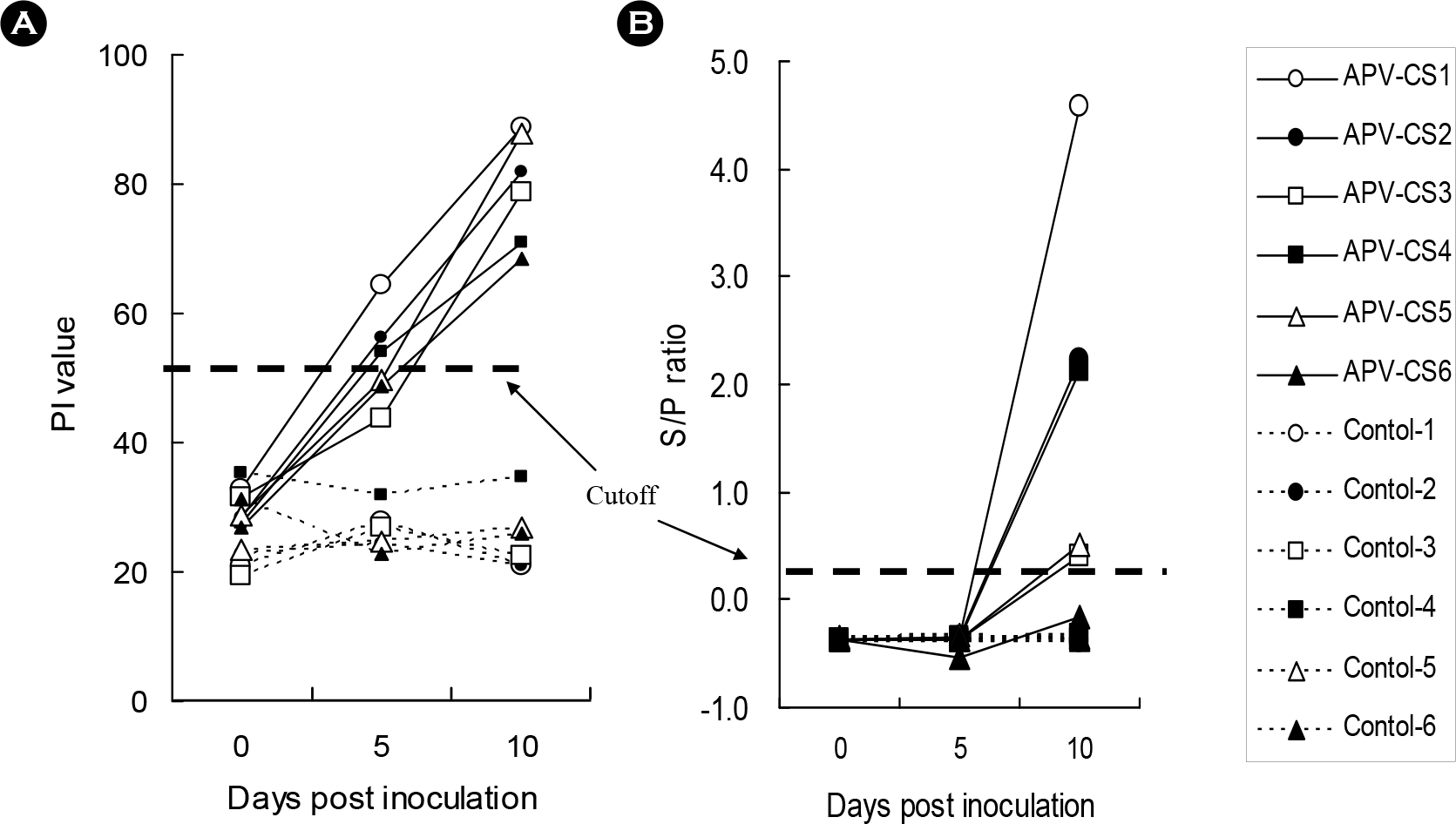 | Figure 5.Detection of anti-aMPV antibodies in experimentally aMPV (subtype B)-infected chickens by a competitive ELISA and comparison with IDEXX-ELISA. Cutoff values of C-ELISA (A) and IDEXX-ELISA are PI value of 50 and S/P ratio of 0.2, respectively. |
Table 1.
Detection of antibodies to aMPV in clinical samples of infected chicken farms in Korea by competitive ELISA
| Farma | SHSb | Age (w.o) | Serum | Yolk | ||
|---|---|---|---|---|---|---|
| C-ELISA | IDEXX-ELISA | C-ELISA | IDEXX-ELISA | |||
| KS (B) | Yes | 32 | 38/40 (95%)c | 39/40 (98%) | NT | NT |
| SJ (B) | Yes | 30 | NT | NT | 9/15 (60%) | 10/15 (67%) |
| SD (A) | Yes | 35 | NT | NT | 12/30 (40%) | 11/30 (37%) |
| HM (A)d | No | 16 | 9/10 (90%)∗ | 4/10 (40%)∗ | NT | NT |
| 20 | 10/10 (100%) | 10/10 (100%) | NT | NT | ||
| PA (B) | Yes | 30 | NT | NT | 21/30 (70%) | 23/30 (77%) |
| CS (B) | No | 65 | 18/20 (90%) | 19/20 (95%) | 29/30 (97%) | 28/30 (93%) |
a Letter in parenthesis represents subtype of aMPV at the time of diagnosis. aMPV subtype was determined by phylogenetic analysis based on the sequence of G protein gene of aMPV (7).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download