Abstract
Foot-and-mouth disease (FMD) is a highly contagious, virally induced disease of cloven-hoofed animals. FMD-affected countries have suffered from a serious economic impact due to their decreased participation in the international livestock trade. Currently, disease control measures include inhibition of susceptible animal movement, slaughter of infected and susceptible in-contact animals, disinfection, and vaccination with an inactivated whole virus antigen. Researchers have attempted to develop new FMD vaccines to overcome the limitations of the current inactivated vaccine as well as new antivirals to more rapidly induce a protective response. In this study, we discuss the most effective novel FMD vaccines and antiviral strategies that are currently being studied. The vaccine research using subunits, synthetic peptides, DNA, cytokine-enhanced DNA, recombinant empty capsids, chimeric viruses, genetically engineered attenuated viruses, recombinant viral vectors, self-replicating DNA and transgenic plants expressing virus proteins is part of a trend towards novel FMD vaccine development. The antiviral methods using RNA interference (RNAi), RNAi-based recombinant adenoviruses and Lpro or 3Dpol inhibitors represent the current replication-inhibiting medicine used to control FMD.
Go to : 
REFERENCES
1). Balamurugan V., Kumar RM., Suryanarayana VV. Past and present vaccine development strategies for the control of foot-and-mouth disease. Acta Virol. 2004. 48:201–14.
3). Paton DJ., Valarcher JF., Bergmann I., Matlho OG., Zakharov VM., Palma EL, et al. Selection of foot and mouth disease vaccine strains–a review. Rev Sci Tech. 2005. 24:981–93.
4). Cottam EM., Wadsworth J., Shaw AE., Rowlands RJ., Goatley L., Maan S, et al. Transmission pathways of foot-and-mouth disease virus in the United Kingdom in 2007. PLoS Pathog. 2008. 4:e1000050.

5). Sobrino F., Saiz M., Jimenez-Clavero MA., Nunez JI., Rosas MF., Baranowski E, et al. Foot-and-mouth disease virus: a long known virus, but a current threat. Vet Res. 2001. 32:1–30.

6). Rodriguez LL., Grubman MJ. Foot and mouth disease virus vaccines. Vaccine. 2009. ;27 Suppl. 4:D90–4.

7). Barteling SJ. Development and performance of inactivated vaccines against foot and mouth disease. Rev Sci Tech. 2002. 21:577–588.

8). Capstick PB., Garland AJ., Chapman WG., Masters RC. Production of foot-and-mouth disease virus antigen from BHK 21 clone 13 cells grown and infected in deep suspension cultures. Nature. 1965. 205:1135–6.

9). Bahnemann HG. Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch Virol. 1975. 47:47–56.

10). Oem JK., Chang BS., Joo HD., Yang MY., Kim GJ., Park JY, et al. Development of an epitope-blocking-enzyme-linked immunosorbent assay to differentiate between animals infected with and vaccinated against foot-and-mouth disease virus. J Virol Methods. 2007. 142:174–81.

11). Ishimaru D., Sa-Carvalho D., Silva JL. Pressure-inactivated FMDV: a potential vaccine. Vaccine. 2004. 22:2334–9.

12). Mowat GN., Barr DA., Bennett JH. The development of an attenuated foot-and-mouth disease virus vaccine by modification and cloning in tissue cultures of BHK21 cells. Arch Gesamte Virusforsch. 1969. 26:341–54.
13). Saiz M., Nunez JI., Jimenez-Clavero MA., Baranowski E., Sobrino F. Foot-and-mouth disease virus: biology and prospects for disease control. Microbes Infect. 2002. 4:1183–92.
14). de Los Santos T., de Avila Botton S., Weiblen R., Grubman MJ. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J Virol. 2006. 80:1906–14.

15). Mason PW., Piccone ME., McKenna TS., Chinsangaram J., Grubman MJ. Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology. 1997. 227:96–102.

16). Chinsangaram J., Mason PW., Grubman MJ. Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine. 1998. 16:1516–22.

17). Ward G., Rieder E., Mason PW. Plasmid DNA encoding replicating foot-and-mouth disease virus genomes induces antiviral immune responses in swine. J Virol. 1997. 71:7442–7.

18). McKenna TS., Lubroth J., Rieder E., Baxt B., Mason PW. Receptor binding site-deleted foot-and-mouth disease (FMD) virus protects cattle from FMD. J Virol. 1995. 69:5787–90.

19). Rieder E., Bunch T., Brown F., Mason PW. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J Virol. 1993. 67:5139–45.

20). Zibert A., Maass G., Strebel K., Falk MM., Beck E. Infectious foot-and-mouth disease virus derived from a cloned full-length cDNA. J Virol. 1990. 64:2467–73.

21). Xin A., Li H., Li L., Liao D., Yang Y., Zhang N, et al. Genome analysis and development of infectious cDNA clone of a virulence-attenuated strain of foot-and-mouth disease virus type Asia 1 from China. Vet Microbiol. 2009. 138:273–80.

22). Piccone ME., Pauszek S., Pacheco J., Rieder E., Kramer E., Rodriguez LL. Molecular characterization of a foot-and-mouth disease virus containing a 57-nucleotide insertion in the 3′ untranslated region. Arch Virol. 2009. 154:671–6.
23). Rodriguez Pulido M., Sobrino F., Borrego B., Saiz M. Attenuated foot-and-mouth disease virus RNA carrying a deletion in the 3′ noncoding region can elicit immunity in swine. J Virol. 2009. 83:3475–85.

24). Van Rensburg HG., Mason PW. Construction and evaluation of a recombinant foot-and-mouth disease virus: implications for inactivated vaccine production. Ann N Y Acad Sci. 2002. 969:83–7.
25). Grubman MJ., Mason PW. Prospects, including time-frames, for improved foot and mouth disease vaccines. Rev Sci Tech. 2002. 21:589–600.

26). Kleid DG., Yansura D., Small B., Dowbenko D., Moore DM., Grubman MJ, et al. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981. 214:1125–9.

27). Verdaguer N., Mateu MG., Andreu D., Giralt E., Domingo E., Fita I. Structure of the major antigenic loop of foot-and-mouth disease virus complexed with a neutralizing antibody: direct involvement of the Arg-Gly-Asp motif in the interaction. EMBO J. 1995. 14:1690–6.

28). Rodriguez LL., Barrera J., Kramer E., Lubroth J., Brown F., Golde WT. A synthetic peptide containing the consensus sequence of the G-H loop region of foot-and-mouth disease virus type-O VP1 and a promiscuous T-helper epitope induces peptide-specific antibodies but fails to protect cattle against viral challenge. Vaccine. 2003. 21:3751–6.

29). Meloen RH., Casal JI., Dalsgaard K., Langeveld JP. Synthetic peptide vaccines: success at last. Vaccine. 1995. 13:885–6.

30). Taboga O., Tami C., Carrillo E., Nunez JI., Rodriguez A., Saiz JC, et al. A large-scale evaluation of peptide vaccines against foot-and-mouth disease: lack of solid protection in cattle and isolation of escape mutants. J Virol. 1997. 71:2606–14.

31). Wang CY., Chang TY., Walfield AM., Ye J., Shen M., Chen SP, et al. Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine. 2002. 20:2603–10.

32). Park JH., Kim SJ., Oem JK., Lee KN., Kim YJ., Kye SJ, et al. Enhanced immune response with foot and mouth disease virus VP1 and interleukin-1 fusion genes. J Vet Sci. 2006. 7:257–62.

33). Li Z., Yi Y., Yin X., Zhang Z., Liu J. Expression of foot-and-mouth disease virus capsid proteins in silkworm-baculovirus expression system and its utilization as a subunit vaccine. PLoS One. 2008. 3:e2273.

34). Wong HT., Cheng SC., Chan EW., Sheng ZT., Yan WY., Zheng ZX, et al. Plasmids encoding foot-and-mouth disease virus VP1 epitopes elicited immune responses in mice and swine and protected swine against viral infection. Virology. 2000. 278:27–35.

35). Aggarwal N., Barnett PV. Antigenic sites of foot-and-mouth disease virus (FMDV): an analysis of the specificities of anti-FMDV antibodies after vaccination of naturally susceptible host species. J Gen Virol. 2002. 83:775–82.

36). Beard C., Ward G., Rieder E., Chinsangaram J., Grubman MJ., Mason PW. Development of DNA vaccines for foot-and-mouth disease, evaluation of vaccines encoding replicating and non-replicating nucleic acids in swine. J Biotechnol. 1999. 73:243–9.

37). Cedillo-Barron L., Foster-Cuevas M., Belsham GJ., Lefevre F., Parkhouse RM. Induction of a protective response in swine vaccinated with DNA encoding foot-and-mouth disease virus empty capsid proteins and the 3D RNA polymerase. J Gen Virol. 2001. 82:1713–24.
38). Wang X., Zhang X., Kang Y., Jin H., Du X., Zhao G, et al. Interleukin-15 enhance DNA vaccine elicited mucosal and systemic immunity against foot and mouth disease virus. Vaccine. 2008. 26:5135–44.

39). Lu H., Huo X., Zhang Y., Zheng M., Ma M., Zhang H, et al. Enhancing effects of the chemical adjuvant levamisole on the DNA vaccine pVIR-P12A-IL18-3C. Microbiol Immunol. 2008. 52(9):440–6.
40). Shao HJ., Chen L., Su YB. DNA fragment encoding human IL-1beta 163-171 peptide enhances the immune responses elicited in mice by DNA vaccine against foot-and-mouth disease. Vet Res Commun. 2005. 29:35–46.
41). Dory D., Remond M., Beven V., Cariolet R., Zientara S., Jestin A. Foot-and-mouth disease virus neutralizing antibodies production induced by pcDNA3 and Sindbis virus based plasmid encoding FMDV P1-2A3C3D in swine. Antiviral Res. 2009. 83:45–52.

42). Zhang A., Jin H., Zhang F., Ma Z., Tu Y., Ren Z, et al. Effects of multiple copies of CpG on DNA vaccination. DNA Cell Biol. 2005. 24:292–8.

43). Zhang S., Guo YJ., Sun SH., Wang KY., Wang KH., Zhang Y, et al. DNA vaccination using bacillus Calmette-Guerin-DNA as an adjuvant to enhance immune response to three kinds of swine diseases. Scand J Immunol. 2005. 62:371–7.

44). Fan H., Tong T., Chen H., Guo A. Immunization of DNA vaccine encoding C3d-VP1 fusion enhanced protective immune response against foot-and-mouth disease virus. Virus Genes. 2007. 35:347–57.

45). Pacheco JM., Brum MC., Moraes MP., Golde WT., Grubman MJ. Rapid protection of cattle from direct challenge with foot-and-mouth disease virus (FMDV) by a single inoculation with an adenovirus-vectored FMDV subunit vaccine. Virology. 2005. 337:205–9.

46). Pena L., Moraes MP., Koster M., Burrage T., Pacheco JM., Segundo FD, et al. Delivery of a foot-and-mouth disease virus empty capsid subunit antigen with nonstructural protein 2B improves protection of swine. Vaccine. 2008. 26:5689–99.

47). Du Y., Jiang P., Li Y., He H., Jiang W., Wang X, et al. Immune responses of two recombinant adenoviruses expressing VP1 antigens of FMDV fused with porcine granulocyte macrophage colony-stimulating factor. Vaccine. 2007. 25:8209–19.

48). Li X., Liu R., Tang H., Jin M., Chen H., Qian P. Induction of protective immunity in swine by immunization with live attenuated recombinant pseudorabies virus expressing the capsid precursor encoding regions of foot-and-mouth disease virus. Vaccine. 2008. 26:2714–22.

49). Ren XG., Xue F., Zhu YM., Tong GZ., Wang YH., Feng JK, et al. Construction of a recombinant BHV-1 expressing the VP1 gene of foot and mouth disease virus and its immunogenicity in a rabbit model. Biotechnol Lett. 2009. 31:1159–65.

50). Ma M., Jin N., Shen G., Zhu G., Liu HJ., Zheng M, et al. Immune responses of swine inoculated with a recombinant fowlpox virus co-expressing P12A and 3C of FMDV and swine IL-18. Vet Immunol Immunopathol. 2008. 121:1–7.

51). Dus Santos MJ., Wigdorovitz A., Trono K., Rios RD., Franzone PM., Gil F, et al. A novel methodology to develop a foot and mouth disease virus (FMDV) peptide-based vaccine in transgenic plants. Vaccine. 2002. 20:1141–7.

52). Pan L., Zhang Y., Wang Y., Wang B., Wang W., Fang Y, et al. Foliar extracts from transgenic tomato plants expressing the structural polyprotein, P1-2A, and protease, 3C, from foot-and-mouth disease virus elicit a protective response in guinea pigs. Vet Immunol Immunopathol. 2008. 121:83–90.

53). Wu L., Jiang L., Zhou Z., Fan J., Zhang Q., Zhu H, et al. Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine. 2003. 21:4390–8.
54). Yang CD., Liao JT., Lai CY., Jong MH., Liang CM., Lin YL, et al. Induction of protective immunity in swine by recombinant bamboo mosaic virus expressing foot-and-mouth disease virus epitopes. BMC Biotechnol. 2007. 7:62.

55). Mason HS., Ball JM., Shi JJ., Jiang X., Estes MK., Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci U S A. 1996. 93:5335–40.

56). Quattrocchi V., Bianco V., Fondevila N., Pappalardo S., Sadir A., Zamorano P. Use of new adjuvants in an emergency vaccine against foot-and-mouth disease virus: evaluation of conferred immunity. Dev Biol (Basel). 2004. 119:481–97.
57). Cloete M., Dungu B., Van Staden LI., Ismail-Cassim N., Vosloo W. Evaluation of different adjuvants for foot-and-mouth disease vaccine containing all the SAT serotypes. Onderstepoort J Vet Res. 2008. 75:17–31.

58). Capozzo AV., Burke DJ., Fox JW., Bergmann IE., La Torre JL., Grigera PR. Expression of foot and mouth disease virus non-structural polypeptide 3ABC induces histone H3 cleavage in BHK21 cells. Virus Res. 2002. 90:91–9.

59). Moffat K., Knox C., Howell G., Clark SJ., Yang H., Belsham GJ, et al. Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C. J Virol. 2007. 81:1129–39.

60). Grubman MJ., Moraes MP., Diaz-San Segundo F., Pena L., de los Santos T. Evading the host immune response: how foot-and-mouth disease virus has become an effective pathogen. FEMS Immunol Med Microbiol. 2008. 53:8–17.

61). Kim SM., Lee KN., Park JY., Ko YJ., Joo YS., Kim HS, et al. Therapeutic application of RNA interference against foot-and-mouth disease virus in vitro and in vivo. Antiviral Res. 2008. 80:178–84.
62). Chen W., Yan W., Du Q., Fei L., Liu M., Ni Z, et al. RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J Virol. 2004. 78:6900–7.

63). Liu M., Chen W., Ni Z., Yan W., Fei L., Jiao Y, et al. Cross-inhibition to heterologous foot-and-mouth disease virus infection induced by RNA interference targeting the conserved regions of viral genome. Virology. 2005. 336:51–9.

64). Kahana R., Kuznetzova L., Rogel A., Shemesh M., Hai D., Yadin H, et al. Inhibition of foot-and-mouth disease virus replication by small interfering RNA. J Gen Virol. 2004. 85:3213–7.

65). Chen W., Liu M., Jiao Y., Yan W., Wei X., Chen J, et al. Adenovirus-mediated RNA interference against foot-and-mouth disease virus infection both in vitro and in vivo. J Virol. 2006. 80:3559–66.
66). de Avila Botton S., Brum MC., Bautista E., Koster M., Weiblen R., Golde WT, et al. Immunopotentiation of a foot-and-mouth disease virus subunit vaccine by interferon alpha. Vaccine. 2006. 24:3446–56.

67). Kleina LG., Grubman MJ. Antiviral effects of a thiol protease inhibitor on foot-and-mouth disease virus. J Virol. 1992. 66:7168–75.

Go to : 
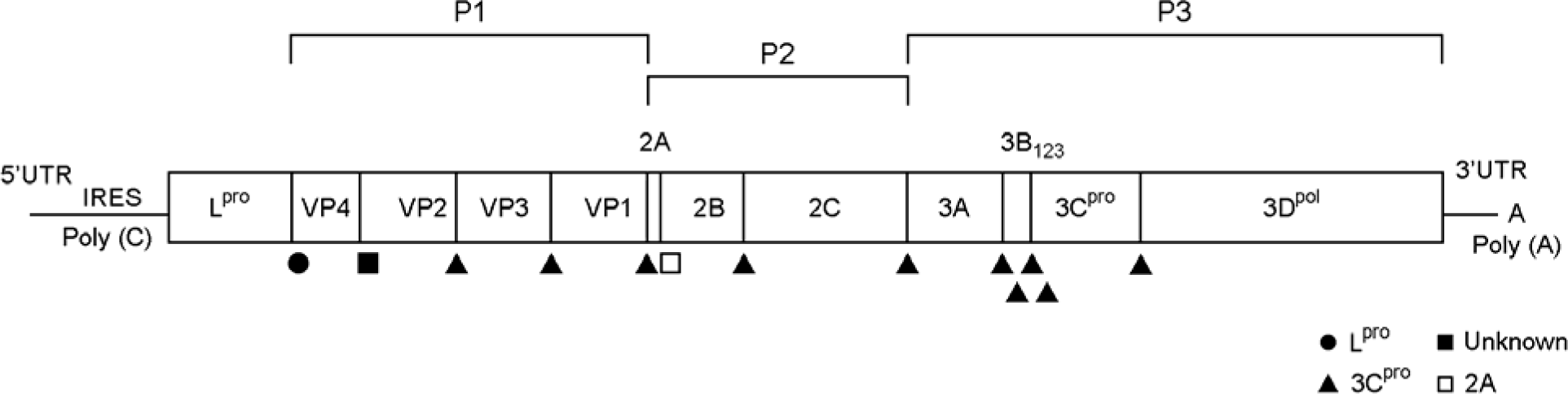 | Figure 1.Schematic diagram of foot-and-mouth disease virus-genome (25). The symbols below the protein-encoding regions identify the proteases responsible for cleavage of the viral polyprotein. |
Table 1.
Characteristics of various FMD vaccine types (5, 25)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download