Abstract
Avian metapneumovirus (AMPV) is an emerging pathogen causing respiratory and reproductive illness in poultry worldwide. To demonstrate the presence of AMPV in domestic chickens in Korea, we attempted to isolate AMPV from affected chickens. A cytopathic agent was isolated using chicken tracheal ring culture from dead chickens from a broiler breeder farm with reduced egg production in Korea. This agent, termed SC1509 strain, subsequently passed in Vero cells with distinct cytopathic effects. The SC1509 strain was confirmed as avian metapneumovirus (AMPV) using both RT-PCR test and monoclonal antibody-based immunofluorescence assay. Sequence analysis based on the G glycoprotein revealed that the SC1509 strain had 22.5 to 96.0% nucleotide sequence identity and 11.1 to 92.7% predicted amino acid sequence identity with previously published AMPV strains, particularly with the highest sequence homology (95.8 to 96% for nucleotides and 92.2 to 92.7% for amino acids) to European strains belonging to genotype B. The SC1509 strain was phylogenetically clustered with genotype B viruses, confirming that the SC1509 strain belongs to genotype B. This is the first report of genotype B avian metapneumovirus from chickens in Korea.
Go to : 
REFERENCES
1). Jones RC. Avian pneumovirus infection: Questions still unanswered. Avian Pathol. 1996. 25:639–48.

2). Office International des Epizooties. Chapter 2.3.15. Turkey rhinotracheitis (avian metapneumovirus). Office International des Epizooties. Manual of diagnostic tests and vaccines for terrestrial animals. 6th ed.Paris: Office International des Epizooties;2008. p. 590–8.
3). Van den Hoogen BG., de Jong JC., Groen J., Kuiken T., de Groot R., Fouchier RA., Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001. 7:719–24.

4). Cook JK., Cavanagh D. Detection and differentiation of avian pneumoviruses (metapneumoviruses). Avian Pathol. 2002. 31:117–32.

5). Njenga MK., Lwamba HM., Seal BS. Metapneumoviruses in birds and humans. Virus Res. 2003. 91:163–9.

6). Bäyon-Auboyer MH., Arnauld C., Toquin D., Eterradossi N. Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup. J Gen Virol. 2000. 81:2723–33.

7). Lwamba HC., Alvarez R., Wise MG., Yu Q., Halvorson D., Njenga MK., Seal BS. Comparison of the full-length genome sequence of avian metapneumovirus subtype C with other paramyxoviruses. Virus Res. 2005. 107:83–92.
8). Banet-Noach C., Simanov L., Perk S. Characterization of Israeli avian metapneumovirus strains in turkeys and chickens. Avian Pathol. 2005. 34:220–6.

9). Lu YS., Shien YS., Tsai HJ., Tseng CS., Lee SH., Lin DF. Swollen head syndrome in Taiwan-isolation of an avian pneumovirus and serological survey. Avian Pathol. 1994. 23:169–74.

10). Mase M., Yamaguchi S., Tsukamoto K., Imada T., Imai K., Nakamura K. Presence of avian pneumovirus subtypes A and B in Japan. Avian Dis. 2003. 47:481–4.

11). Owoade AA., Ducatez MF., Hübschen JM., Sausy A., Chen H., Guan Y., Muller CP. Avian metapneumovirus subtype A in China and subtypes A and B in Nigeria. Avian Dis. 2008. 52:502–6.

12). Jones RC., Naylor CJ., Bradbury JM., Savage CE., Worthington K., Williams RA. Isolation of a turkey rhinotracheitis-like virus from broiler breeder chickens in England. Vet Rec. 1991. 129:509–10.
13). Pattison M., Chettle N., Randall CJ., Wyeth PJ. Observations on swollen head syndrome in broiler and broiler breeder chickens. Vet Rec. 1989. 125:229–31.

14). Buys SB., Du Preez JH., Els HJ. The isolation and attenuation of a virus causing rhinotracheitis in turkeys in South Africa. Onderstepoort J Vet Res. 1989. 56:87–98.
15). Bennett RS., Nezworski J., Velayudhan BT., Nagaraja KV., Zeman DH., Dyer N., Graham T., Lauer DC., Njenga MK., Halvorson DA. Evidence of avian pneumovirus spread beyond Minnesota among wild and domestic birds in central North America. Avian Dis. 2004. 48:902–8.

16). Seal BS. Avian pneumoviruses and emergence of a new type in the United States of America. Anim Health Res Rev. 2000. 1:67–72.

17). Turpin EA., Stallknecht DE., Slemons RD., Zsak L., Swayne DE. Evidence of avian metapneumovirus subtype C infection of wild birds in Georgia, South Carolina, Arkansas and Ohio, USA. Avian Pathol. 2008. 37:343–51.
18). Toquin D., Bäyon-Auboyer MH., Eterradossi N., Jestin V. Isolation of a pneumovirus from a Muscovy duck. Vet Rec. 1999. 145:680.
19). Kim JH., Song CS., Sung HW., Mo IP., Kwon JH., Kim KS., Kim SH. Outbreak of swollen head syndrome in a broiler breeder farm and serological survey of avian pneumovirus. In: Proceedings of the Korean Society of Veterinary Science, 36th meeting, Kwangju, Korea: Korean Society of Veterinary Science. 1992. p.25.
20). Kim JE., Hwang JY., Bae DR., Sung MS., Kim ST., Kim SY. Examination of seroprevalence and detection of avian pneumovirus from layer hens in Gyeongbuk province. Korean J Vet Serv. 2007. 30:43–9.
21). Kim ST., Kim SK., Cho MH., Kim YH. Serological survey of avian pneumovirus infection in laying hens of Gyeongbuk province. Korean J Vet Serv. 2003. 26:51–6.
22). Lee JW., Shon KR., Park KS., Kim YT., Kim CC., Han KS., Lee HM., Song HJ. Serological survey of avian pneumovirus and reovirus in breeders of Jeonbuk province. Korean J Vet Serv. 2006. 29:9–18.
23). Park JB., Cha SY., Park YM., Zhao DD., Song HJ., Jang HK. Recently epidemiological survey of the viral diseases of broiler chickens in Jeonbuk province from 2005 to 2007. Korean J Vet Serv. 2008. 31:43–55.
24). Bäyon-Auboyer MH., Jestin V., Toquin D., Cherbonnel M., Eterradossi N. Comparison of F-, G- and N-based RT-PCR protocols with conventional virological procedures for the detection and typing of turkey rhinotracheitis virus. Arch Virol. 1999. 144:1091–109.

25). Gough RE., Alexander DJ., Wyeth PJ. Avian rhinotrachetitis (pneumovirus). Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM, editors. editors.A laboratory manual for the isolation and identification of avian pathogens. 4th ed. Kennett Square: American Association of Avian Pathologists;1998. p. 164–7.
26). Jeon WJ., Kim BH., Jung BY., An DJ., Yi CH., Jang H., Chung GS. Application of a PCR method for the detection of Mycoplasma in veterinary live viral vaccines. Kor J Microbiol. 2005. 41:269–74.
27). Mase M., Tsukamoto K., Imai K., Yamaguchi S. Phylogenetic analysis of avian infectious bronchitis virus strains isolated in Japan. Arch Virol. 2004. 149:2069–78.

28). Reed LJ., Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938. 27:493–7.
29). Alkhalaf AN., Ward LA., Dearth RN., Saif YM. Pathogenicity, transmissibility, and tissue distribution of avian pneumovirus in turkey poults. Avian Dis. 2002. 46:650–9.

30). Aung YH., Liman M., Neumann U., Rautenschlein S. Reproducibility of swollen sinuses in broilers by experimental infection with avian metapneumovirus subtypes A and B of turkey origin and their comparative pathogenesis. Avian Pathol. 2008. 37:65–74.
31). Cook JK., Chesher J., Orthel F., Woods MA., Orbell SJ., Baxendale W., Huggins MB. Avian pneumovirus infection of laying hens: experimental studies. Avian Pathol. 2000. 29:545–56.

32). Hess M., Huggins MB., Mudzamiri R., Heincz U. Avian metapneumovirus excretion in vaccinated and non-vaccinated specified pathogen free laying chickens. Avian Pathol. 2004. 33:35–40.

33). Shin HJ., McComb B., Back A., Shaw DP., Halvorson DA., Nagaraja KV. Susceptibility of broiler chicks to infection by avian pneumovirus of turkey origin. Avian Dis. 2000. 44:797–802.

34). Giraud P., Bennejean G., Guittet M., Toquin D. Turkey rhinotracheitis in France: preliminary investigations on a ciliostatic virus. Vet Rec. 1986. 119:606–7.
35). McDougall JS., Cook JK. Turkey rhinotracheitis: preliminary investigations. Vet Rec. 1986. 118:206–7.

36). Wilding GP., Baxter-Jones C., Grant M. Ciliostatic agent found in rhinotracheitis. Vet Rec. 1986. 118:735.

37). Wyeth PJ., Gough RE., Chettle N., Eddy R. Preliminary observations on a virus associated with turkey rhinotracheitis. Vet Rec. 1986. 119:139.

38). Panigrahy B., Senne DA., Pedersen JC., Gidlewski T., Edson RK. Experimental and serologic observations on avian pneumovirus (APV/turkey/Colorado/97) infection in turkeys. Avian Dis. 2000. 44:17–22.

39). Shin HJ., Cameron KT., Jacobs JA., Turpin EA., Halvorson DA., Goyal SM., Nagaraja KV., Kumar MC., Lauer DC., Seal BS., Njenga MK. Molecular epidemiology of subgroup C avian pneumoviruses isolated in the United States and comparison with subgroup a and B viruses. J Clin Microbiol. 2002. 40:1687–93.

40). Lee E., Song MS., Shin JY., Lee YM., Kim CJ., Lee YS., Kim H., Choi YK. Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market. Virus Res. 2007. 128:18–25.

41). Chiang SJ., Dar A., Goyal SM., Nagaraja KV., Halvorson D., Kapur V. Isolation of avian pneumovirus in QT-35 cells. Vet Rec. 1998. 143:596.
Go to : 
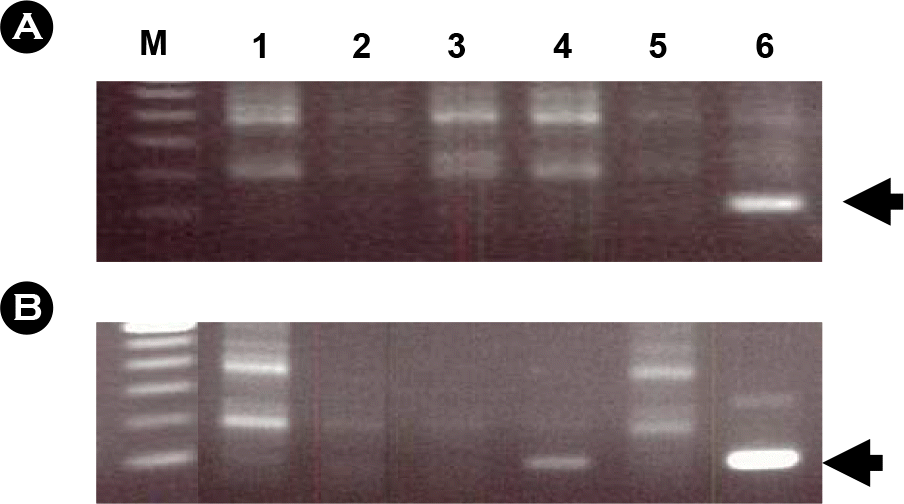 | Figure 1.Detection of AMPV RNA in dead chickens using RT-PCR. Total RNA was isolated from nasopharyngeal swabs (A) or nasal turbinate tissues (B). Lane M, molecular marker; lanes l~6, dead chickens tested. The arrow represents the expected size of PCR products amplified by PCR. |
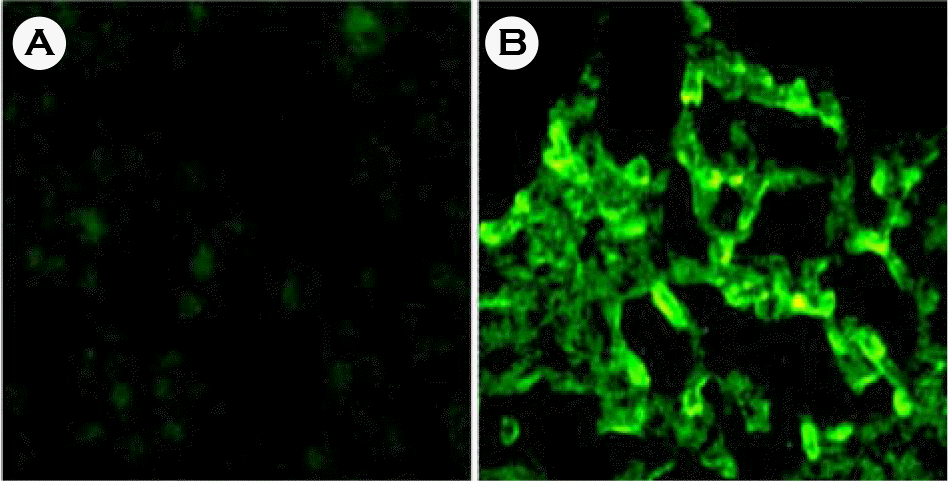 | Figure 2.Immunofluorescent staining of AMPV (SC1509 strain)-infected Vero cells using AMPV-specific monoclonal antibody 12.2.1. A: Mock-infected Vero cells, B: AMPV-infected Vero cells. |
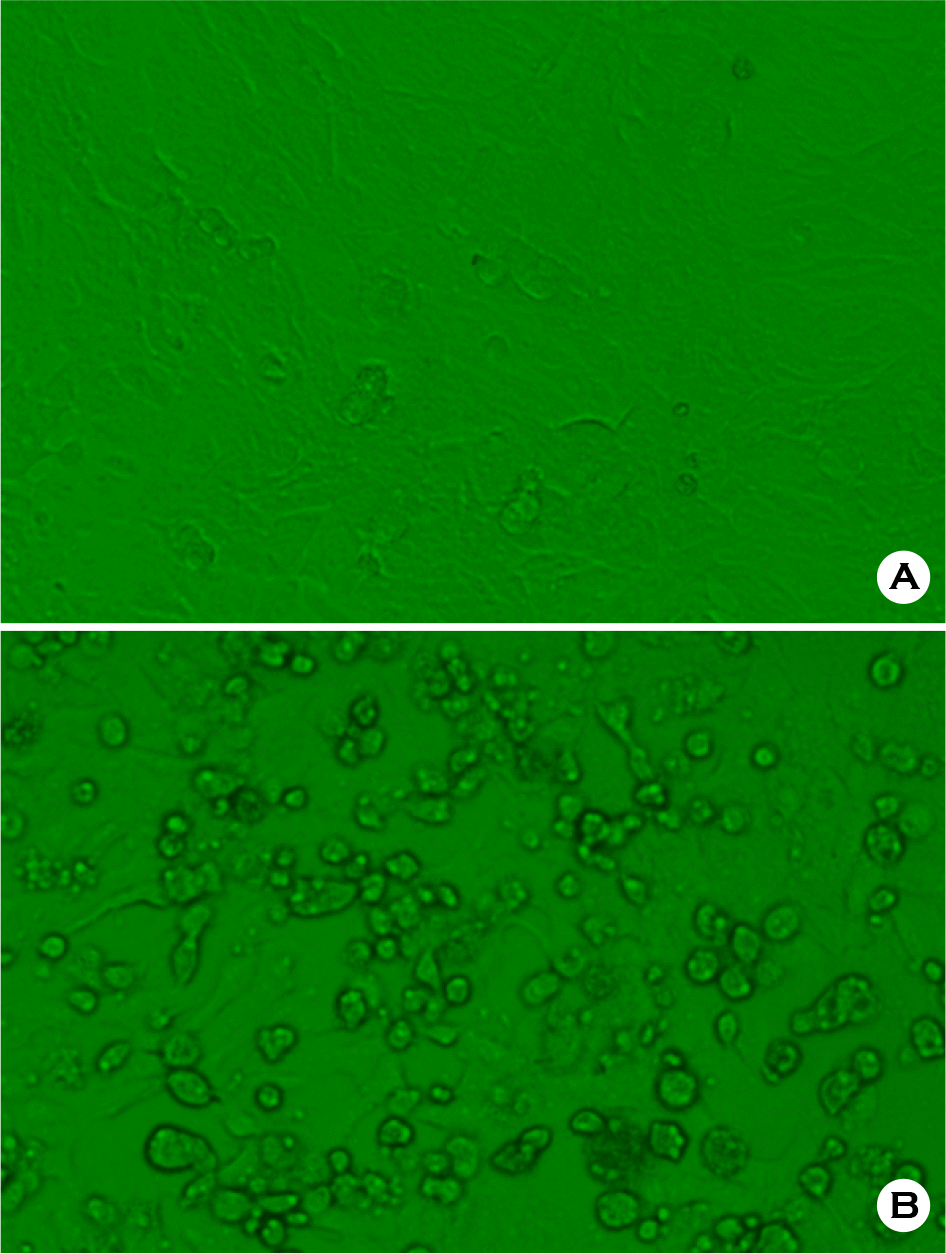 | Figure 3.Cytopathic effects of AMPV SC1509 strain in Vero cells. A: Mock-infected Vero cells, B: AMPV-infected Vero cells. |
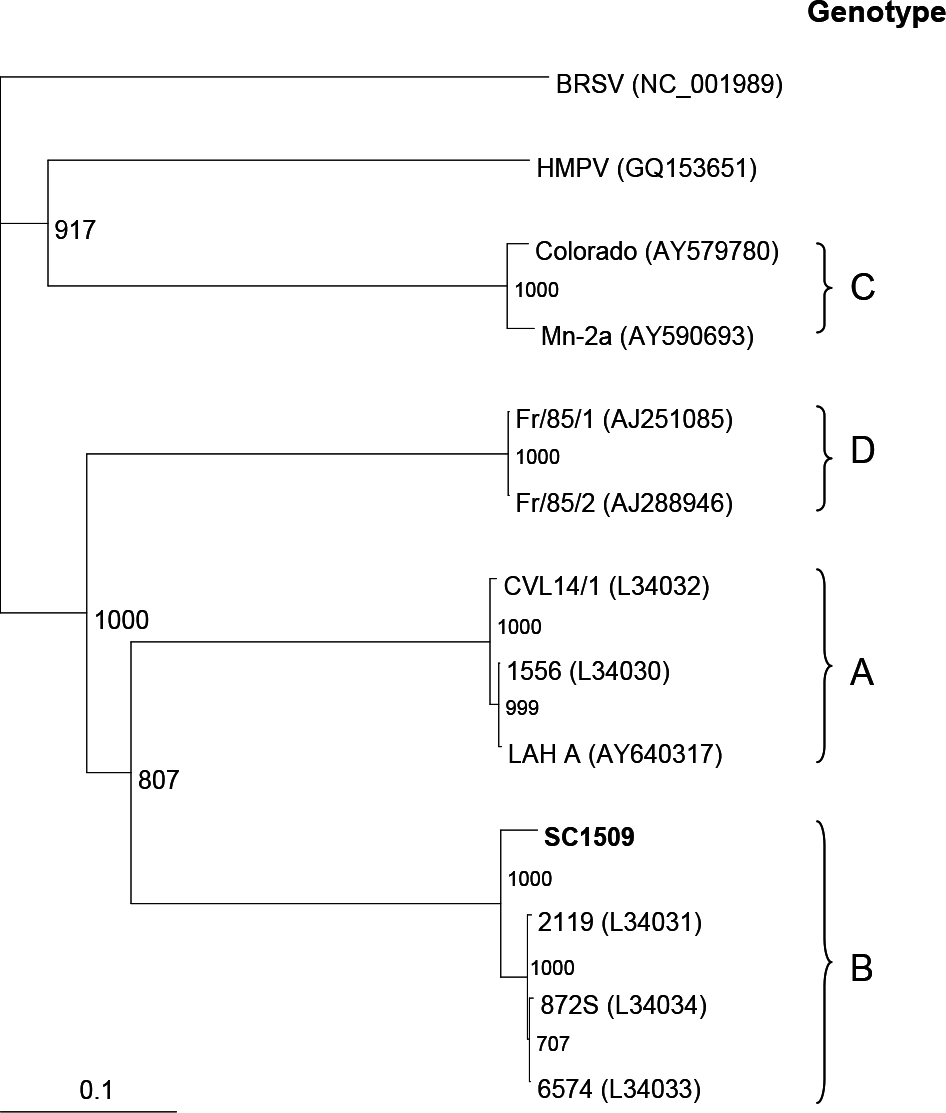 | Figure 4.Phylogenetic anlaysis of AMPV SC1509 strain. Nucleotide sequences were analyzed based on the complete G coding region. AMPV field isolate from this study is shown in bold. Designation in parenthesis represents accession number of reference strains obtained from the GenBank database. The provisional designations including genotypes are indicated on the right. |
Table 1.
Comparison of nucleic acid and deduced amino acid sequences of the G protein of AMPV SC1509 strain with other strains of AMPV




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download