Abstract
A survey was performed in Korea to monitor the prevalence of five bovine arboviruses [Akabane virus, Aino virus, Chuzan virus, bovine ephemeral fever (BEF) virus, and Ibaraki virus] in arthropod vectors, such as Culicoides species. To determine the possible applications of survey data in annual monitoring and warning systems in Korea, we examined the prevalence of bovine arboviruses in arthropod vectors using RT-PCR. To compare the sensitivity and specificity of virus detection, nested PCR was also performed in parallel for all five viruses. Using the RT-PCR, the detection limits were at least up to 101.5, 102.8, 102.0, 101.8, and 104.0 TCID50/ml for Akabane virus, Aino virus, Chuzan virus, BEF virus, and Ibaraki virus, respectively. When nested PCR was performed using 1 μl of PCR product, the detection limits were increased, to 100.05, 101.8, 101.0, 100.008, and 102.0 TCID50/ml for Akabane virus, Aino virus, Chuzan virus, BEF virus, and Ibaraki virus, respectively. Thus, nested PCR increased the sensitivity of the virus detection limit by 1~2 log. We pooled 30~40 mosquitoes in one sample. We collected 113 samples in 2006, 135 samples in 2007, and 100 samples in 2008. Among these samples, Chuzan virus and BEF virus genes were detected at a range between 0.82% and 1.19%, and Akabane virus, Aino virus, and Ibaraki virus genes were detected at less than 0.20%. These data may provide some insight into future epidemiological studies of bovine arboviral diseases in Korea.
Go to : 
REFERENCES
1). Cybinski DH., St George TD., Paull NI. Antibodies to Akabane virus in Australia. Aust Vet J. 1978. 54:1–3.

2). Inaba Y., Kurogi H., Omori T. Letter: Akabane disease: epizootic abortion, premature birth, stillbirth and congenital arthrogryposis-hydranencephaly in cattle, sheep and goats caused by Akabane virus. Aust Vet J. 1975. 51:584–5.
3). Coverdale OR., Cybinski DH., St George TD. Congenital abnormalities in calves associated with Akabane virus and Aino virus. Aust Vet J. 1978. 54:151–2.

4). Haziroglu R., Haritani M., Narita M. Demonstration of Akabane virus antigen in experimentally infected mice using immunoperoxidase method. Nippon Juigaku Zasshi. 1987. 49:133–5.

5). Hartley WJ., Wanner RA., Della-Porta AJ., Snowdon WA. Serological evidence for the association of Akabane virus with epizootic bovine congenital arthrogryposis and hydranencephaly syndromes in New South Wales. Aust Vet J. 1975. 51:103–4.

7). Matumoto M. [Akabane disease and Akabane virus]. Uirusu. 1980. 30:1–10.
8). Mellor PS., Jennings DM., Braverman Y., Boorman J. Infection of Israeli Culicoides with African horse sickness, blue tongue and akabane viruses. Acta Virol. 1981. 25:401–7.
9). Ishibashi K., Shirakawa H., Uchinuno Y., Ogawa T. Seroprevalence survey of Aino virus infection in dairy cattle of Fukuoka, Japan in 1990. J Vet Med Sci. 1995. 57:1–4.

10). Miura Y., Inaba Y., Tsuda T., Tokuhisa S., Sato K., Akashi H., Matumoto M. A survey of antibodies to arthropodborne viruses in Indonesian cattle. Nippon Juigaku Zasshi. 1982. 44:857–63.

11). Tsuda T., Yoshida K., Ohashi S., Yanase T., Sueyoshi M., Kamimura S, et al. Arthrogryposis, hydranencephaly and cerebellar hypoplasia syndrome in neonatal calves resulting from intrauterine infection with Aino virus. Vet Res. 2004. 35:531–8.

12). Cybinski DH., St George TD. A survey of antibody to Aino virus in cattle and other species in Australia. Aust Vet J. 1978. 54:371–3.

13). Uchinuno Y., Noda Y., Ishibashi K., Nagasue S., Shirakawa H., Nagano M., Ohe R. Isolation of Aino virus from an aborted bovine fetus. J Vet Med Sci. 1998. 60:1139–40.

14). Kim YH., Kwon CH., Yang DK. Development of inactivated vaccine for Akabane, Aino and Chuzan Disease. NVRQS Annual Research Report. 2005. 962–75.
15). Cybinski DH., Zakrzewski H. A dual infection of a bull with Akabane and Aino viruses. Aust Vet J. 1983. 60:283.

16). Fukuyoshi S., Takehara Y., Takahashi K., Mori R. The incidence of antibody to Aino virus in animals and humans in Fukuoka. Jpn J Med Sci Biol. 1981. 34:41–3.

17). NVRI. Isolation of Aino virus from malformed calves in Kyung-Book Province. NVRI Case Report. 1997.
18). Burgess GW., Chenoweth PJ. Mid-piece abnormalities in bovine semen following experimental and natural cases of bovine ephemeral fever. Br Vet J. 1975. 131:536–44.

19). Burgess GW., Spradbrow PB. Studies on the pathogenesis of bovine ephemeral fever. Aust Vet J. 1977. 53:363–8.

20). Goto Y., Miura Y., Kono Y. Serologic evidence for the etiologic role of Chuzan virus in an epizootic of congenital abnormalities with hydranencephaly-cerebellar hypoplasia syndrome of calves in Japan. Am J Vet Res. 1988. 49:2026–9.
21). Goto Y., Miura Y., Kono Y. Epidemiological survey of an epidemic of congenital abnormalities with hydranencephaly-cerebellar hypoplasia syndrome of calves occurring in 1985/86 and seroepidemiological investigations on Chuzan virus, a putative causal agent of the disease, in Japan. Nippon Juigaku Zasshi. 1988. 50:405–13.

22). Miura Y., Goto Y., Kubo M., Kono Y. Isolation of Chuzan virus, a new member of the Palyam subgroup of the genus Orbivirus, from cattle and Culicoides oxystoma in Japan. Am J Vet Res. 1988. 49:2022–5.
23). Miura Y., Kubo M., Goto Y., Kono Y. Hydranencephaly-cerebellar hypoplasia in a newborn calf after infection of its dam with Chuzan virus. Nippon Juigaku Zasshi. 1990. 52:689–94.

24). Kurogi H., Matumoto M. Serological comparison of Kagoshima and Chuzan viruses of Palyam serogroup orbivirus isolated in Japan. Kitasato Arch Exp Med. 1989. 62:199–201.
25). Della-Porta AJ., Brown F. The physico-chemical characterization of bovine ephemeral fever virus as a member of the family Rhabdoviridae. J Gen Virol. 1979. 44:99–112.
26). Nandi S., Negi BS. Bovine ephemeral fever: a review. Comp Immunol Microbiol Infect Dis. 1999. 22:81–91.

27). Combs GP. Bovine ephemeral fever. Proc Annu Meet U S Anim Health Assoc. 1978. 29–35.
28). Heuschele WP. Bovine ephemeral fever. I. Characteristics of the causative virus. Arch Gesamte Virusforsch. 1970. 30:195–202.
29). Heuschele WP., Johnson DC. Bovine ephemeral fever. II. Responses of cattle to attenuated and virulent virus. Proc Annu Meet U S Anim Health Assoc. 1969. 73:185–95.
30). Walker PJ. Bovine ephemeral fever in Australia and the world. Curr Top Microbiol Immunol. 2005. 292:57–80.

31). Uren MF., St George TD., Kirkland PD., Stranger RS., Murray MD. Epidemiology of bovine ephemeral fever in Australia 1981-1985. Aust J Biol Sci. 1987. 40:125–36.

32). Walker PJ., Cybinski DH. Bovine ephemeral fever and rhabdoviruses endemic to Australia. Aust Vet J. 1989. 66:398–400.

33). Tzipori S., Spradbrow PB. Development and behaviour of a strain of bovine ephemeral fever virus with unusual host range. J Comp Pathol. 1974. 84:1–8.

34). Tzipori S., Spradbrow PB. The effect of bovine ephemeral fever virus on the bovine foetus. Aust Vet J. 1975. 51:64–6.

35). Suzuki Y., Saito Y., Nakagawa S. Double-stranded RNA of Ibaraki virus. Virology. 1977. 76:670–4.

36). Suzuki Y., Nakagawa S., Namiki M., Saito Y. RNA and protein of Ibaraki virus. Kitasato Arch Exp Med. 1978. 51:61–71.
37). Omori T., Inaba Y., Morimoto T., Tanaka Y., Kono M. Ibaraki virus, an agent of epizootic disease of cattle resembling bluetongue. II. Isolation of the virus in bovine cell culture. Jpn J Microbiol. 1969. 13:159–68.
38). Inaba Y., Tanaka Y., Ishii S., Morimoto T., Sato K. Ibaraki virus, an agent of epizootic disease of cattle resembling bluetongue. IV. Physicochemical and serological properties of the virus. Jpn J Microbiol. 1970. 14:351–60.
39). Omori T., Inaba Y., Morimoto T., Tanaka Y., Ishitani R. Ibaraki virus, an agent of epizootic disease of cattle resembling bluetongue. I. Epidemiologic, clinical and pathologic observations and experimental transmission to calves. Jpn J Microbiol. 1969. 13:139–57.
40). Bak UB LC., Cheong CK., Hwang WS., Cho MR. Outbreaks of Akabane disease of cattle in Korea. Korean J Vet Res. 1980. 20:65–78.
41). Park B. An outbreak of Chuzan disease in Korea and the immunogenicity of binary ethylenimine-treated chuzan virus vaccine in cattle. Korean J Vet Publ Hlth. 1993. 17:301–5.
42). Song GC., Kang BJ. Development of vaccine and diagnostic methods for bovine ephemeral fever. NVRQS Annual Research Report. 1970. 61–8.
43). Park BK. Characterization of Ibaraki virus isolated in Korea. NVRQS Annual Report. 1993.
44). Mellor PS., Boorman J., Baylis M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol. 2000. 45:307–40.

45). Cho HC., Chong CS. Notes on biting midges of the genus culicoides from South Korea: with special reference to unrecorded species and distribution. Kisaengchunghak Chapchi. 1974. 2:45–75.
46). Park BK. Monitoring of bovine ephemeral fever, Ibaraki disease and Akabane disease in Korea. NVRQS Annual Report. 1992.
47). Allingham PG., Standfast HA. An investigation of transovarial transmission of Akabane virus in Culicoides brevitarsis. Aust Vet J. 1990. 67:273–4.

48). Jennings M., Mellor PS. Culicoides: biological vectors of Akabane virus. Vet Microbiol. 1989. 21:125–31.

49). Kurogi H., Akiba K., Inaba Y., Matumoto M. Isolation of Akabane virus from the biting midge Culicoides oxystoma in Japan. Vet Microbiol. 1987. 15:243–8.
50). Murray MD. Akabane epizootics in New South Wales: evidence for long-distance dispersal of the biting midge Culicoides brevitarsis. Aust Vet J. 1987. 64:305–8.
51). St George TD., Standfast HA., Cybinski DH. Isolations of akabane virus from sentinel cattle and Culicoides brevitarsis. Aust Vet J. 1978. 54:558–61.
52). Theodoridis A., Nevill EM., Els HJ., Boshoff ST. Viruses isolated from Culicoides midges in South Africa during unsuccessful attempts to isolate bovine ephemeral fever virus. Onderstepoort J Vet Res. 1979. 46:191–8.
53). Venter GJ., Hamblin C., Paweska JT. Determination of the oral susceptibility of South African livestock-associated biting midges, Culicoides species, to bovine ephemeral fever virus. Med Vet Entomol. 2003. 17:133–7.

54). REED M. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938. 27:493–7.
55). Akashi H., Onuma S., Nagano H., Ohta M., Fukutomi T. Detection and differentiation of Aino and Akabane Simbu serogroup bunyaviruses by nested polymerase chain reaction. Arch Virol. 1999. 144:2101–9.

56). Cho JJ. Development of antigen detection method for bovine ephemeral fever virus. NVRQS Annual Report. 1997.
57). Uchinuno Y., Ito T., Goto Y., Miura Y., Ishibashi K., Itou T., Sakai T. Differences in Ibaraki virus RNA segment 3 sequences from three epidemics. J Vet Med Sci. 2003. 65:1257–63.

58). Stram Y., Kuznetzova L., Guini M., Rogel A., Meirom R., Chai D., Yadin H., Brenner J. Detection and quantitation of akabane and aino viruses by multiplex real-time reverse-transcriptase PCR. J Virol Methods. 2004. 116:147–54.

59). Ministry of Food A, Forestry and Fisheries, Republic of Korea. Act on the Prevention of Cotagious Animal Diseases. 2008.
60). Ministry of Food, Agriculture, Forestry and Fisheries, Republic of Korea. National Project for the Prevention of Contagious Animal Diseases. 2009.
61). Disease Diagnostic Center NVRQS. Monitoring of Seroprevalence against five bovine arboviral diseases in Korea in 2008. 2008.
Go to : 
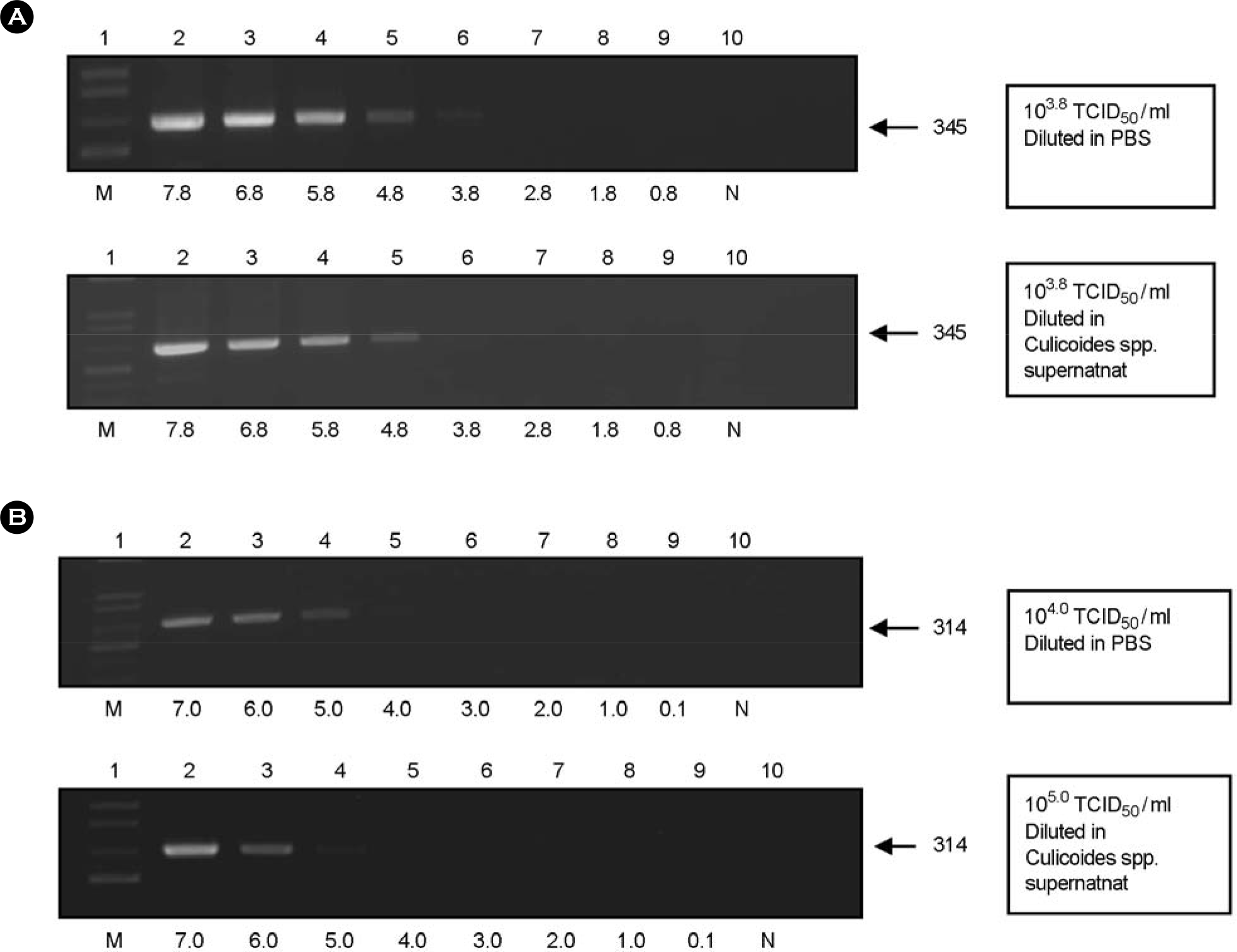 | Figure 1.Detection limits determined by spiking Aino virus and Ibaraki virus into supernatant from Culicoides species. Five microliters of each PCR product from PBS-diluted virus or supernatant from Culicoides species were applied to a 1% agarose gel as follows: (A) Aino virus. Virus diluted in PBS (upper). 1: Marker, 2~9: diluted Aino virus, 10: negative control. Virus diluted in Culicoides species supernatant (bottom). 1: Marker, 2~9: diluted Aino virus, 10: negative control (B) Ibaraki virus. Virus diluted in PBS (upper). 1: Marker, 2~9: diluted Ibaraki virus, 10: negative control. Virus diluted in Culicoides species supernatant (bottom). 1: Marker, 2~9: diluted Ibaraki virus, 10: negative control. |
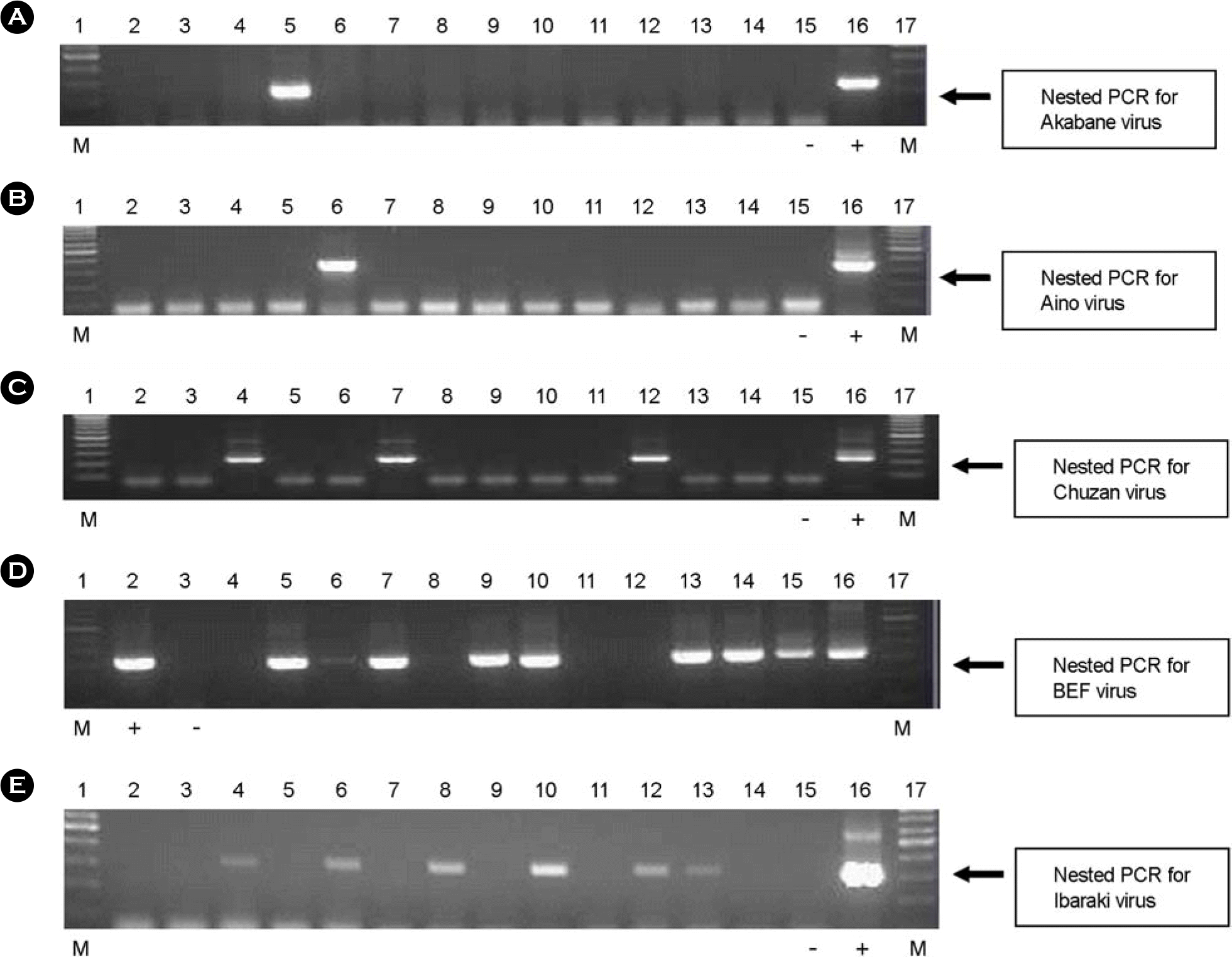 | Figure 2.Prevalence of five bovine arboviral genes in arthropod vectors by nested PCR amplifications. RT-PCR, and subsequently nested PCR, were performed on RNA from pooled Culicoides species supernatant. Five microliters of the product from nested PCR were applied to a 1% agarose gel as follows: (A) nested PCR for Akabane virus. 1: Marker, 2~14: samples (5: Akabane virus positive sample), 15: negative control, 16: positive control, 17: Marker (B) nested PCR for Aino virus. 1: Marker, 2~14: samples (6: Aino virus positive sample), 15: negative control, 16: positive control, 17: Marker (C) nested PCR for Chuzan virus. 1: Marker, 2~14: samples (4, 7, 12: Chuzan virus positive sample), 15: negative control, 16: positive control, 17: Marker (D) nested PCR for BEF virus. 1: Marker, 2: positive control, 3: negative control, 4~16: samples (5, 6, 7, 9, 10, 13, 14, 15, 16: BEF virus positive sample), 17: Marker (E) nested PCR for Ibaraki virus. 1: Marker, 2~14: samples (4, 6, 8, 10, 12, 13: Ibaraki virus positive sample), 15: negative control, 16: positive control, 17: Marker. |
Table 1.
The oilognucleotide primers for RT-PCR and nested PCR
Table 2.
Detection limits of five bovine arboviral genes by RT-PCR and nested PCR
Table 3.
Detection limits of five bovine arboviral genes by RT-PCR and nested PCR
| Year | No. of samples | No. of positive % (%)a | ||||
|---|---|---|---|---|---|---|
| Akakane | Aino | Chuzan | BEF | Ibaraki | ||
| 0 | 4 | 27 | 25 | 2 | ||
| 2006 | 113 | 0% (0%)∗ | 3.54% (0.10%) | 23.89% (0.68%) | 22.12% (0.63%) | 1.77% (0.05%) |
| 3 | 4 | 48 | 78 | 5 | ||
| 2007 | 135 | 2.22% (0.06%) | 2.96% (0.09%) | 35.56% (1.02%) | 57.78% (1.65%) | 3.70% (0.11%) |
| 2 | 0 | 25 | 42 | 13 | ||
| 2008 | 100 | 2% (0.06%) | 0% (0%) | 25% (0.71%) | 45% (1.20%) | 13% (0.37%) |
| 5 | 8 | 100 | 145 | 20 | ||
| Total | 348 | 1.44% (0.04%) | 2.30% (0.07%) | 28.74% (0.82%) | 41.67% (1.19%) | 5.75% (0.16%) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download