Abstract
REFERENCES
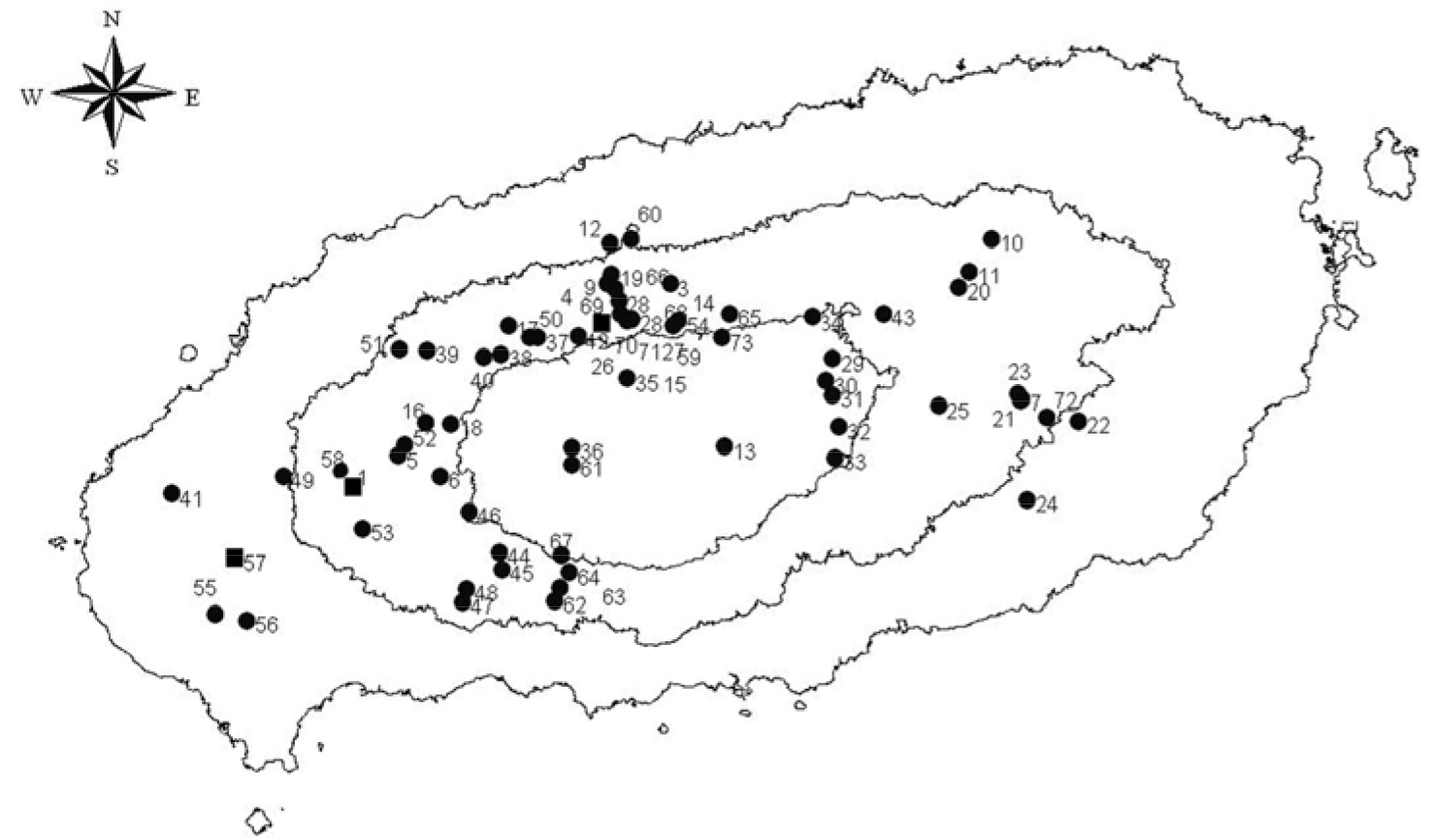 | Figure 1.Map of Jeju Island, Korea showing the geographic distribution of ticks collected from 2007 to 2008. Dots indicate the collection area of the H. longicornis and squares indicate the collection area of H. longicornis and H. flava. |
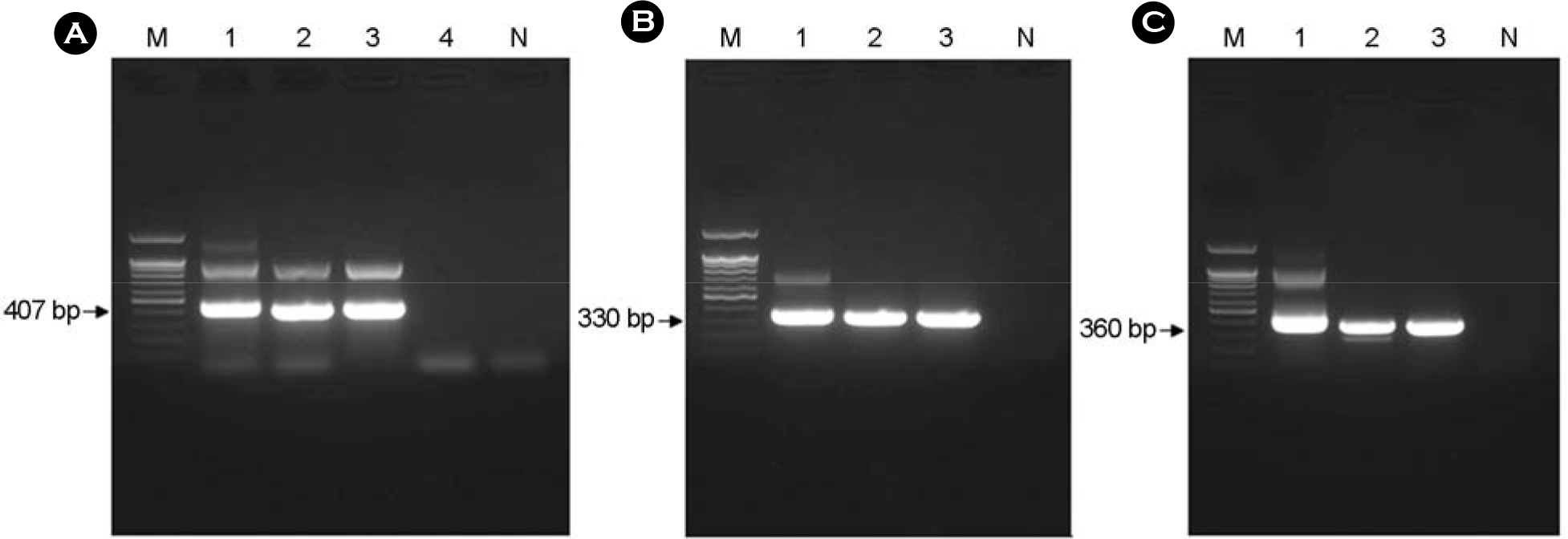 | Figure 2.Electrophoresis analysis on 1.2% agarose gel of DNAs amplified by PCR targeted ompB (panel A), gltA (panel B), and the 17-kDa (panel C) antigen gene. (A) The size of amplified ompB product was about 407 bp. Lane 1, positive control (R. japonica); lane 2~4, each number of the amplified ompB products. (B) The size of amplified gltA product was about 330 bp. Lane 1, positive control (R. japonica); lane 2~3, each number of the amplified gltA products. (C) The size of amplified 17-kDa product was about 360 bp. Lane 1, positive control (R. japonica); lane 2~3, each number of the amplified 17-kDa products. Lane M, 100 bp DNA ladder; lane N, negative control. The number on the left indicates the molecular size (in base pairs) of the amplified PCR products. |
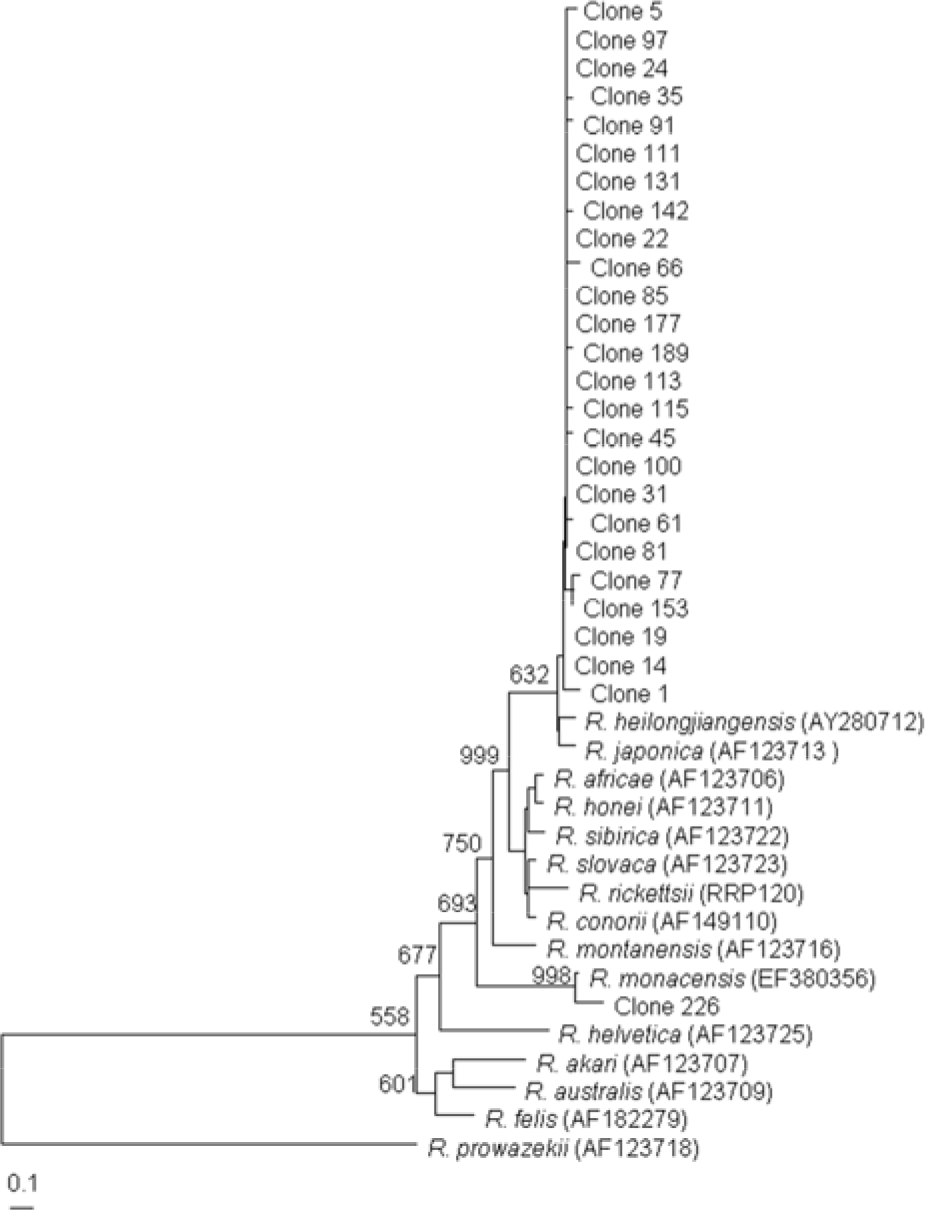 | Figure 3.Dendrogram representing phylogenetic relationships between partial ompB gene sequences (the size of about 407 bp) of various Rickettsial strain and PCR-amplified ompB products from tick. Phylograms were generated by neighbor-joining analysis with 1,000 bootstrapped replicates. |
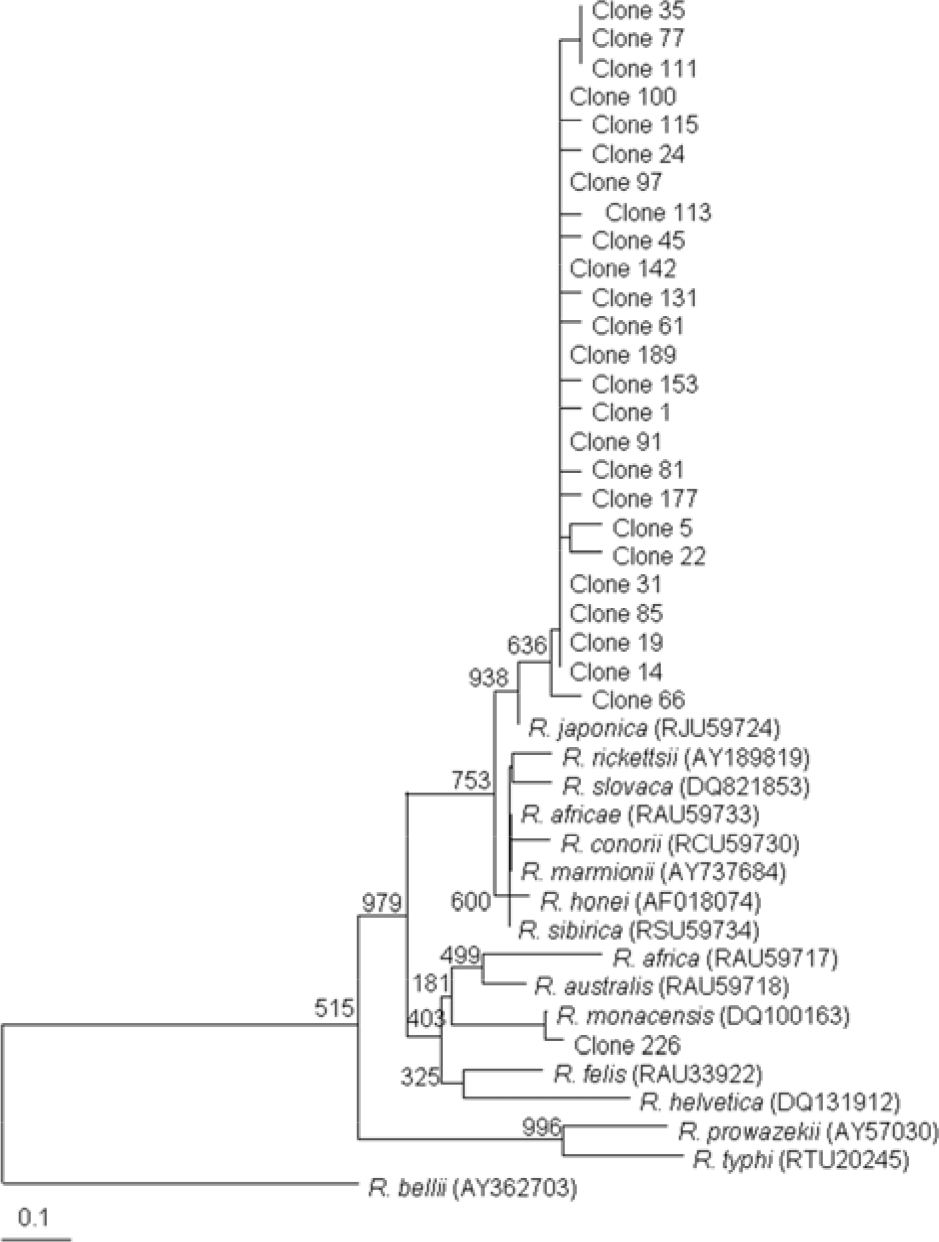 | Figure 4.Dendrogram representing phylogenetic relationships between partial gltA gene sequences (the size of about 330 bp) of various Rickettsial strains and PCR-amplified gltA products from tick. Phylograms were generated by neighbor-joining analysis with 1,000 bootstrapped replicates. |
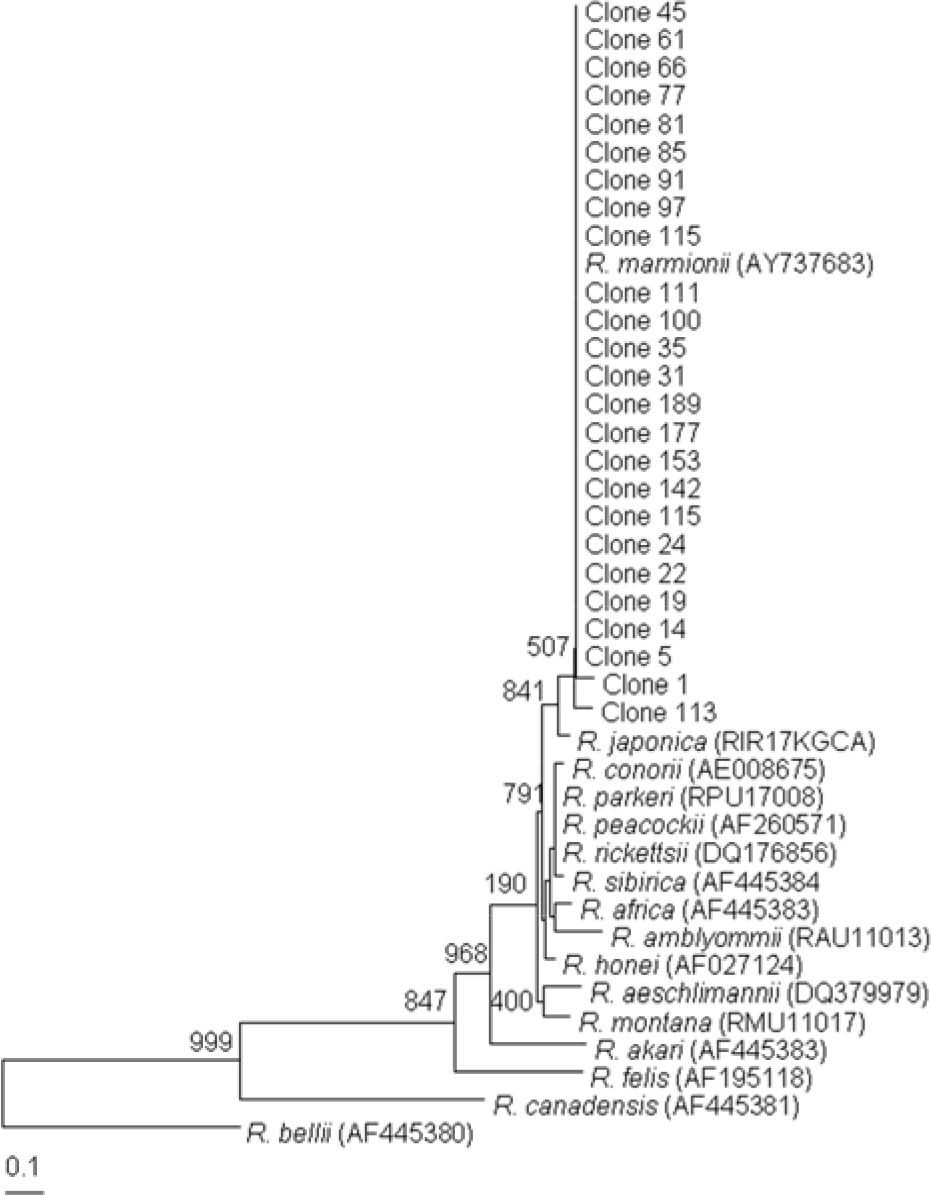 | Figure 5.Dendrogram representing phylogenetic relationships between partial 17-kDa gene sequences (the size of about 360 bp) of various Rickettsial strain and PCR-amplified 17-kDa products from tick. Phylograms were generated by neighbor-joining analysis with 1,000 bootstrapped replicates. |
Table 1.
| Ta rget genea | Primer | Nucleotide sequence (5′→3′) | Products size | PCR condition | References | |||
|---|---|---|---|---|---|---|---|---|
| Denaturation | c Annealingc | Extensionc | Cycles | |||||
| ompB | WJ77 OF | GTAACCGGAAGTAATCGTTTCGTAA | 500 bp | 94 | 54 | 72 | 40 | |
| WJ80b OR | GCTTTATAACCAGCTAAACCACC | |||||||
| WJ79 SFG IF | GTTTAATACGTGCTGCTAACCAA | SFG 407 bp | 94 | 56 | 72 | 35 | 23 | |
| WJ83 TG IF | AAGATCCTTCTGATGTTGCAACA | TG 231 bp | ||||||
| WJ78b SFG/TG IR | GGTTTGGCCCATATACCATAAG | |||||||
| gltA | RpCS.877p OF | GGGGGCCTGCTCACGGCGG | 381 bp | 94 | 52 | 72 | 35 | |
| RpCS.1,258nb OR | ATTGCAAAAAGTACAGTGAACA | |||||||
| RpCS.896p IF | GGCTAATGAAGCAGTGATAA | 330 bp | 94 | 54 | 72 | 30 | ||
| RpCS.1,233nb IR | GCGACGGTATACCCATAGC | |||||||
| 17-kDa | Rr17k. 1p OF | TTTACAAAATTCTAAAACCAT | 540 bp | 94 | 47 | 72 | 35 | |
| Rr17k. 539nb OR | TCAATTCACAACTTGCCATT | |||||||
| Rr17k. 90p IF | GCTCTTGCAACTTCTATGTT | 360 bp | 94 | 47 | 72 | 30 | ||
| Rr17k. 417nb IR | TTTCCGCCTATTACAACTGTT | |||||||
Table 2.
| Species | Stages | Collection | No. ticks | No. pools | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | ||||||||||
| June | July | August | April | May | June | July | August | ||||
| Larvaea | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | |
| H. longicornis | Nymphb | 614 | 223 | 36 | 26 | 130 | 115 | 20 | 9 | 1,173 | 104 |
| Adults malec | 44 | 77 | 14 | 0 | 4 | 33 | 3 | 4 | 179 | 65 | |
| Adults femalec | 49 | 99 | 26 | 0 | 1 | 36 | 8 | 7 | 226 | 77 | |
| Larvaea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| H. flava | Nymphb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adults malec | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Adults femalec | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 2 | |
| Total | 708 | 399 | 79 | 26 | 136 | 184 | 31 | 21 | 1,584 | 250 | |
Table 3.
1, partial ompB of R. africae (AF123706); 2, R. akari (AF123707); 3, R. conorii (AF149110); 4, R. japonica (AB003681); 5, R. monacensis (EF380356); 6, R. sibirica (AF123722); 7, ompB PCR clone 1; 8, clone 5; 9, clone 14; 10, clone 35; 11, clone 45; 12, clone 81; 13, clone 85; 14, clone 97; 15, clone 100; 16, clone 226
Table 4.
1, partial gltA of R. africae (RAU59733); 2, R. akari (RAU59717); 3, R. conorii (RCU59730); 4, R. japonica (RJU59724); 5, R. marmionii (AY737684) 6, R. monacensis (DQ100163); 7, R. sibirica (RSU59734); 8, gltA PCR clone 1; 9, clone 5; 10, clone 14; 11, clone 35; 12, clone 45; 13, clone 81; 14, clone 85; 15, clone 97; 16, clone 100; 17, clone 226
Table 5.
1, partial 17-kDa of R. africae (AF445383); 2, R. akari (AF445383) 3, R. conorii (AE008675); 4, R. japonica (RIR17KGCA); 5, R. marmionii (AY737683); 6, R. monacensis (EF380355) 7, R. sibirica (AF445384); 8, 17-kDa PCR clone 1; 9, clone 5; 10, clone 14; 11, clone 35; 12, clone 45; 13, clone 81; 14, clone 85; 15, clone 97; 16, clone 100




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download