Abstract
The objective of this study was to analyze quantitatively whether Weissella cibaria could affect the proliferation of five periodontopathic bacteria, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum, after incubation for 8~48 h. In addition, by using real-time PCR with a dual-labeled probe, each growth of bacteria was examined under different growth media conditions. The proliferation of periodontopathic bacteria was significantly inhibited by W. cibaria after incubation for 24~48 h (p < 0.05), whereas the growth of W. cibaria was not affected by these pathogenic bacteria. The growth of P. gingivalis, T. forsythia and T. denticola significantly increased in each growth media after incubation for 24 h (p < 0.05), as compared to the culture in mixed growth media. However, no differences in the growth of five periodontopathic bacteria were observed between each growth media and mixed media after incubation for 48 h. The growth and pH of W. cibaria culture significantly were changed in MRS after incubation for 24~48 h (p < 0.05), as compared to the bacterial culture in mixed growth media. The pH of P. gingivalis and F. nucleatum culture significantly was changed in both growth media and mixed media after incubation for 24~48 h (p < 0.05). Our data indicate that W. cibaria significantly inhibits the proliferation of five periodontopathic bacteria and each growth of bacteria is quantitatively analyzed under various media conditions by real-time PCR.
Go to : 
REFERENCES
1). Papapanou PN. Epidemiology of periodontal diseases: an update. J Int Acad Periodontol. 1999. 1:110–6.
2). Socransky SS., Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992. 63:322–31.

3). Wiebe CB., Putnins EE. The periodontal disease classification system of the American Academy of Periodontology: an update. J Can Dent Assoc. 2000. 66:594–7.
4). Poulet PP., Duffaut D., Lodter JP. Metronidazole susceptibility testing of anaerobic bacteria associated with periodontal disease. J Clin Periodontol. 1999. 26:261–3.

5). Roldan S., Herrera D., Santa-Cruz I., O'Connor A., Gonzalez I., Sanz M. Comparative effects of different chlorhexidine mouth-rinse formulations on volatile sulphur compounds and salivary bacterial counts. J Clin Periodontol. 2004. 31:1128–34.

6). Cho MJ., Hong SJ., Choi CH., Jeong SS. Effects of dentifrice containing extract of Galla rhois or Psoralea corylifolia on inhibition of plaque formation. J Kor Acad Dent Health. 2005. 29:141–52.
7). Estafan D., Gultz J., Kaim JM., Khaghany K., Scherer W. Clinical efficacy of an herbal toothpaste. J Clin Dent. 1998. 9:31–3.
8). Çaglar E., Kargul B., Tanboga I. Bacteriotherapy and probiotics' role on oral health. Oral Dis. 2005. 11:131–7.

9). Guarner F., Perdigon G., Corthier G., Salminen S., Koletzko B., Morelli L. Should yoghurt cultures be considered probiotic? Br J Nutr. 2005. 93:783–6.

10). Mandell RL. A longitudinal microbiological investigation of Actinobacillus actinomycetemcomitans and Eikenella corrodens in juvenile periodontitis. Infect Immun. 1984. 45:778–80.
11). Haffajee AD., Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994. 5:78–111.

12). Socransky SS., Haffajee AD., Cugini MA., Smith C., Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998. 25:134–44.

13). Kato H., Yoshida A., Awano S., Ansai T., Takehara T. Quantitative detection of volatile sulfur compound-producing microorganisms in oral specimens using real-time PCR. Oral Dis. 2005. 11:67–71.
14). Sakamoto M., Takeuchi Y., Umeda M., Ishikawa I., Benno Y. Rapid detection and quantification of five periodontopathic bacteria by real-time PCR. Microbiol Immunol. 2001. 45:39–44.

15). Suzuki N., Yoshida A., Nakano Y. Quantitative analysis of multi-species oral biofilms by TaqMan Real-Time PCR. Clin Med Res. 2005. 3:176–85.

16). Yoshida A., Suzuki N., Nakano Y., Oho T., Kawada M., Koga T. Development of a 5′ fluorogenic nuclease-based real-time PCR assay for quantitative detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. J Clin Microbiol. 2003. 41:863–6.
17). Stiles ME., Holzapfel WH. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 1997. 36:1–29.

18). Bjorkroth KJ., Schillinger U., Geisen R., Weiss N., Hoste B., Holzapfel WH., Korkeala HJ., Vandamme P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int J Syst Evol Microbiol. 2002. 52:141–8.
19). Kang MS., Chung J., Kim SM., Yang KH., Oh JS. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006. 40:418–25.
20). Kang MS., Kim BG., Chung J., Lee HC., Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulfur compounds. J Clin Periodontol. 2006. 33:226–32.
21). Kang MS., Choi EK., Choi DH., Ryu SY., Lee HH., Kang HC., Koh JT., Kim OS., Hwang YC., Yoon SJ., Kim SM., Yang KH., Kang IC. Antibacterial activity of pyrrolidine dithiocarbamate. FEMS Microbiol Lett. 2008. 280:250–4.

22). Ohta K., Makinen KK., Loesche WJ. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986. 53:213–20.
23). American Academy of Periodontology. Consensus report. Periodontal diseases: Pathogenesis and microbial factors. Ann Periodontol. 1996. 1:926–32.
24). Lopez NJ. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia in progressive adult periodontitis. J Periodontol. 2000. 71:948–54.
25). Lai CH., Listgarten MA., Shirakawa M., Slots J. Bacteroides forsythus in adult gingivitis and periodontitis. Oral Microbiol Immunol. 1987. 2:152–7.
26). Tanner A., Maiden MF., Macuch PJ., Murray LL., Kent RL Jr. Microbiota of health, gingivitis, and initial periodontitis. J Clin Periodontol. 1998. 25:85–98.

27). Listgarten MA. Electron microscopic observations of the bacterial flora of acute necrotizing ulcerative gingivitis. J Periodontol. 1965. 36:328–39.
28). Simonson LG., Goodman CH., Bial JJ., Morton HE. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988. 56:726–8.
29). Dzink JL., Tanner AC., Haffajee AD., Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985. 12:648–59.

30). Shimazaki Y., Shirota T., Uchida K., Yonemoto K., Kiyohara Y., Iida M., Saito T., Yamashita Y. Intake of dairy products and periodontal disease: the Hisayama Study. J Periodontol. 2008. 79:131–7.

31). Çaglar E., Sandalli N., Twetman S., Kavaloglu S., Ergeneli S., Selvi S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand. 2005. 63:317–20.
32). Çglar E., Cildir SK., Ergeneli S., Sandalli N., Twetman S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand. 2006. 64:314–8.
33). Hatakka K., Ahola AJ., Yli-Knuuttila H., Richardson M., Poussa T., Meurman JH., Korpela R. Probiotics reduce the prevalence of oral Candida in the elderly-a randomized controlled trial. J Dent Res. 2007. 86:125–30.
34). Kang MS., Na HS., Oh JS. Coaggregation ability of Weissella cibaria isolates with Fusobacterium nucleatum and their adhesiveness to epithelial cells. FEMS Microbiol Lett. 2005. 253:323–9.
35). Boutaga K., van Winkelhoff AJ., Vandenbroucke-Grauls CM., Savelkoul PH. Comparison of real-time PCR and culture for detection of Porphyromonas gingivalis in subgingival plaque samples. J Clin Microbiol. 2003. 41:4950–4.
36). Srionnual S., Yanagida F., Lin LH., Hsiao KN., Chen YS. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl Environ Microbiol. 2007. 73:2247–50.
Go to : 
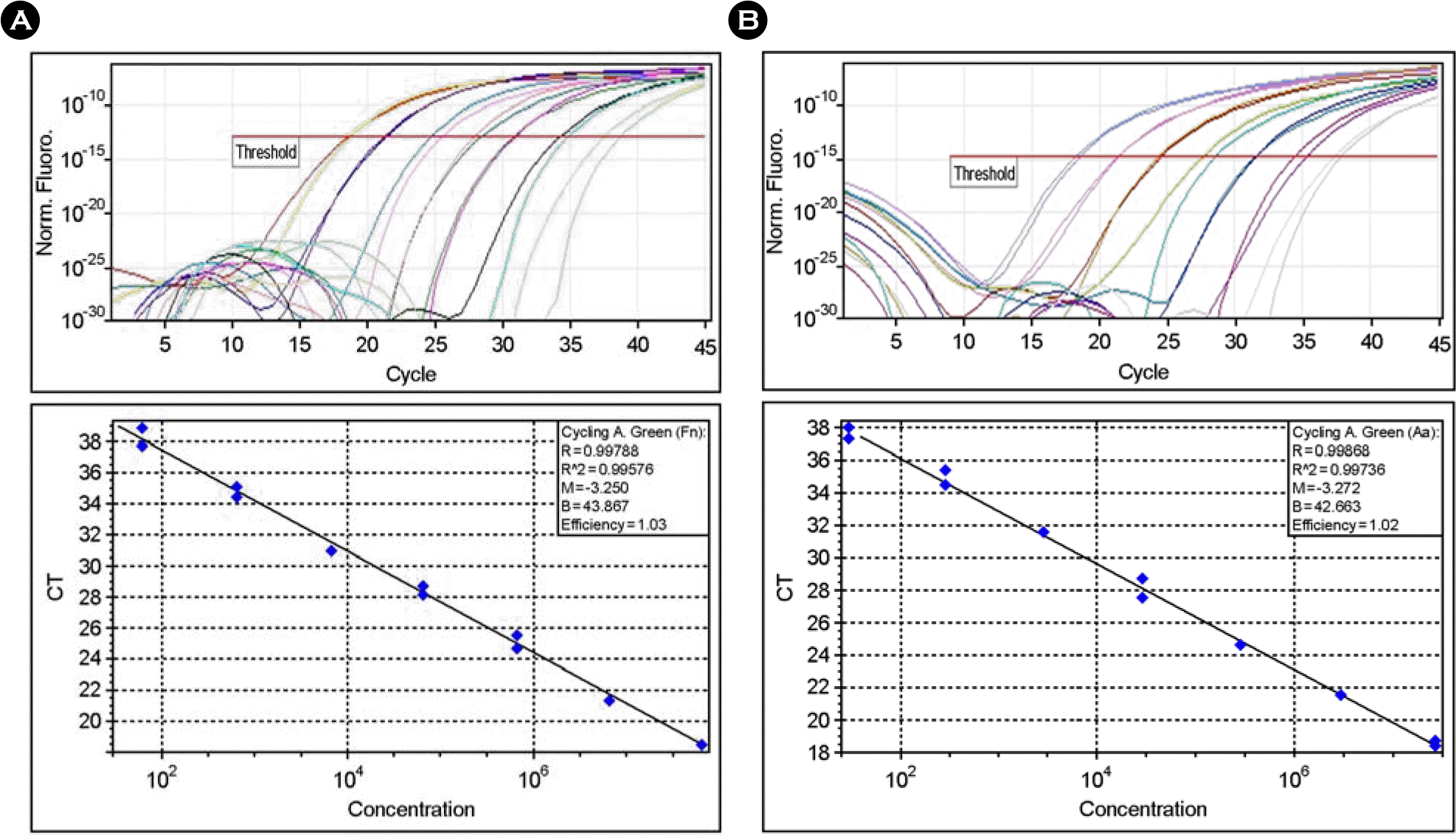 | Figure 1.Amplification of genomic DNA from lysed cells. Serial dilutions of genomic DNA from F. nucleatum (A) or A. actinomycetemcomitans (B) were used as templates for real-time PCR. The threshold fluorescence, or the level at which the threshold cycle was determined, is shown. The standard curves were generated from the amplification plots in the insets (correlation coefficients, 0.996 for F. nucleatum and 0.997 for A. actinomycetemcomitans). CT is the cycle number at which the threshold fluorescence is reached. The linearity with R value is observed from 102 to 108 bacterial cells. |
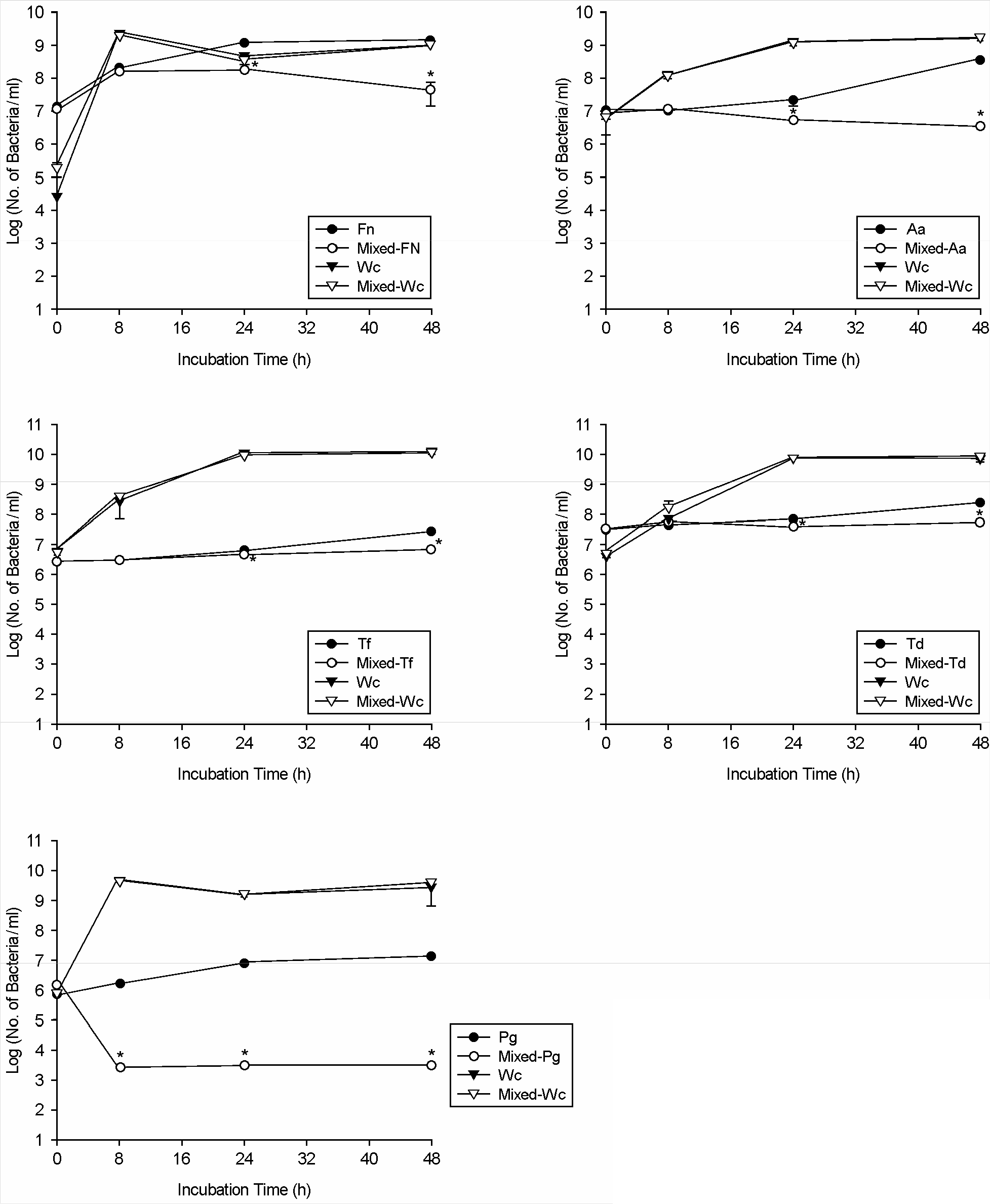 | Figure 2.Quantitative comparison of bacterial cell numbers of W. cibaria and periodontopathic bacteria in the mixed cultures by real-time PCR. Fn, F. nucleatum; Aa, A. actinomycetemcomitans; Tf, T. forsythia; Td, T. denticola; Pg, P. gingivalis; Wc, W. cibaria. ∗p < 0.05 for coculture versus monoculture. Values are means ± standard deviations of three independent experiments. |
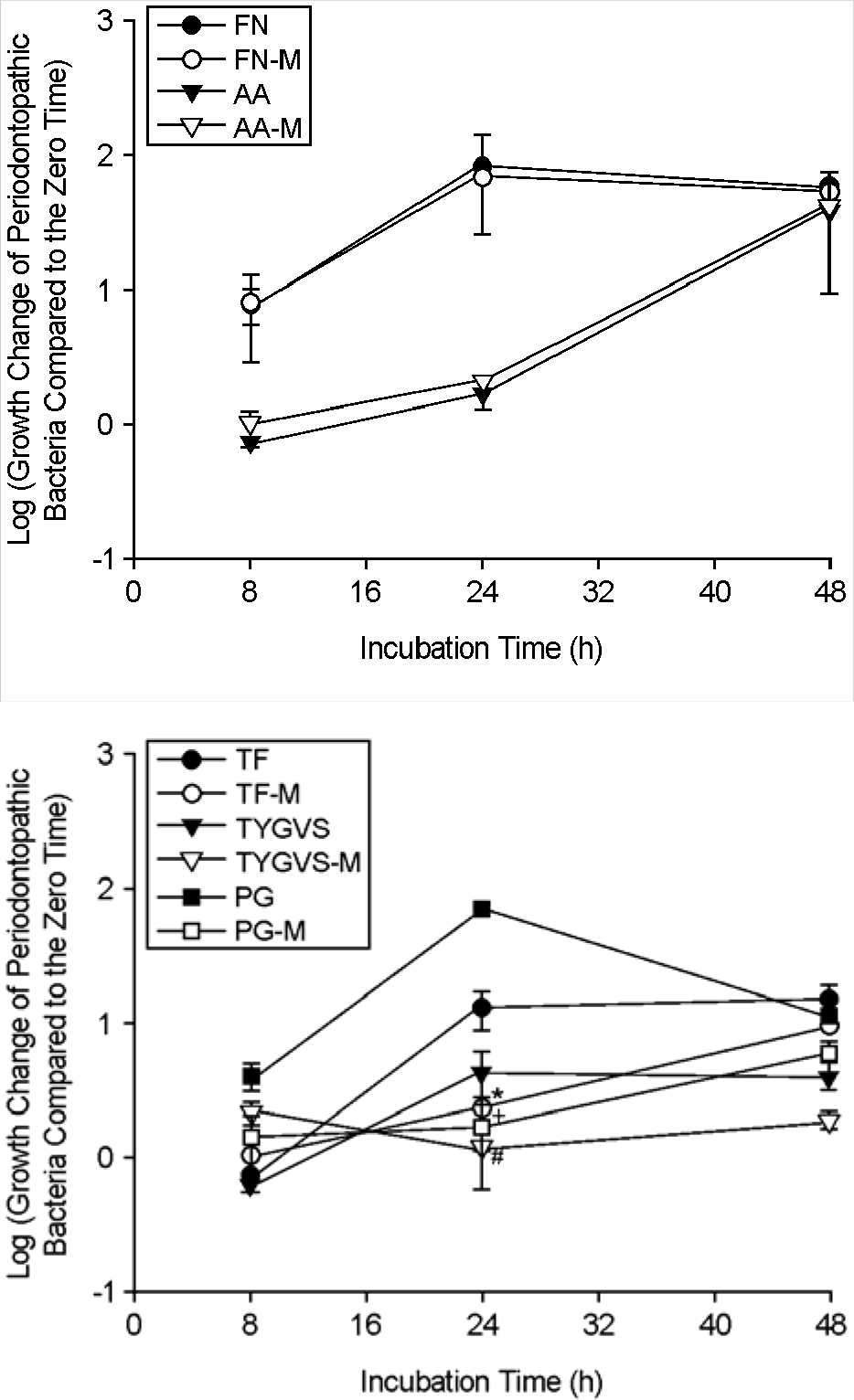 | Figure 3.Comparison of bacterial cell numbers of periodontopathic bacteria over time under different growth media conditions by real-time PCR. The growth change of periodontopathic bacteria compared to the zero time (0 h) was shown as log scale. F. nucleatum was grown in FN (F. nucleatum growth medium) or FN-M (a mixture of equal volume of FN and MRS). A. actinomycetemcomitans was grown in AA (A. actinomycetemcomitans growth medium) or AA-M (a mixture of equal volume of AA and MRS). T. forsythia was grown in TF (T. forsythia growth medium) or TF-M (a mixture of equal volume of TF and MRS). T. denticola was grown in TYGVS (T. denticola growth medium) or TYGVS-M (a mixture of equal volume of TYGVS and MRS). P. gingivalis was grown in PG (P. gingivalis growth medium) or PG-M (a mixture of equal volume of PG and MRS). ∗p < 0.05, T. forsythia culture in TF-M versus T. forsythia culture in TF; #p < 0.05, T. denticola culture in TYGVS-M versus T. denticola culture in TYGVS; +p < 0.05, P. gingivalis culture in PG-M versus P. gingivalis culture in PG. Values are means ± standard deviations of three independent experiments. |
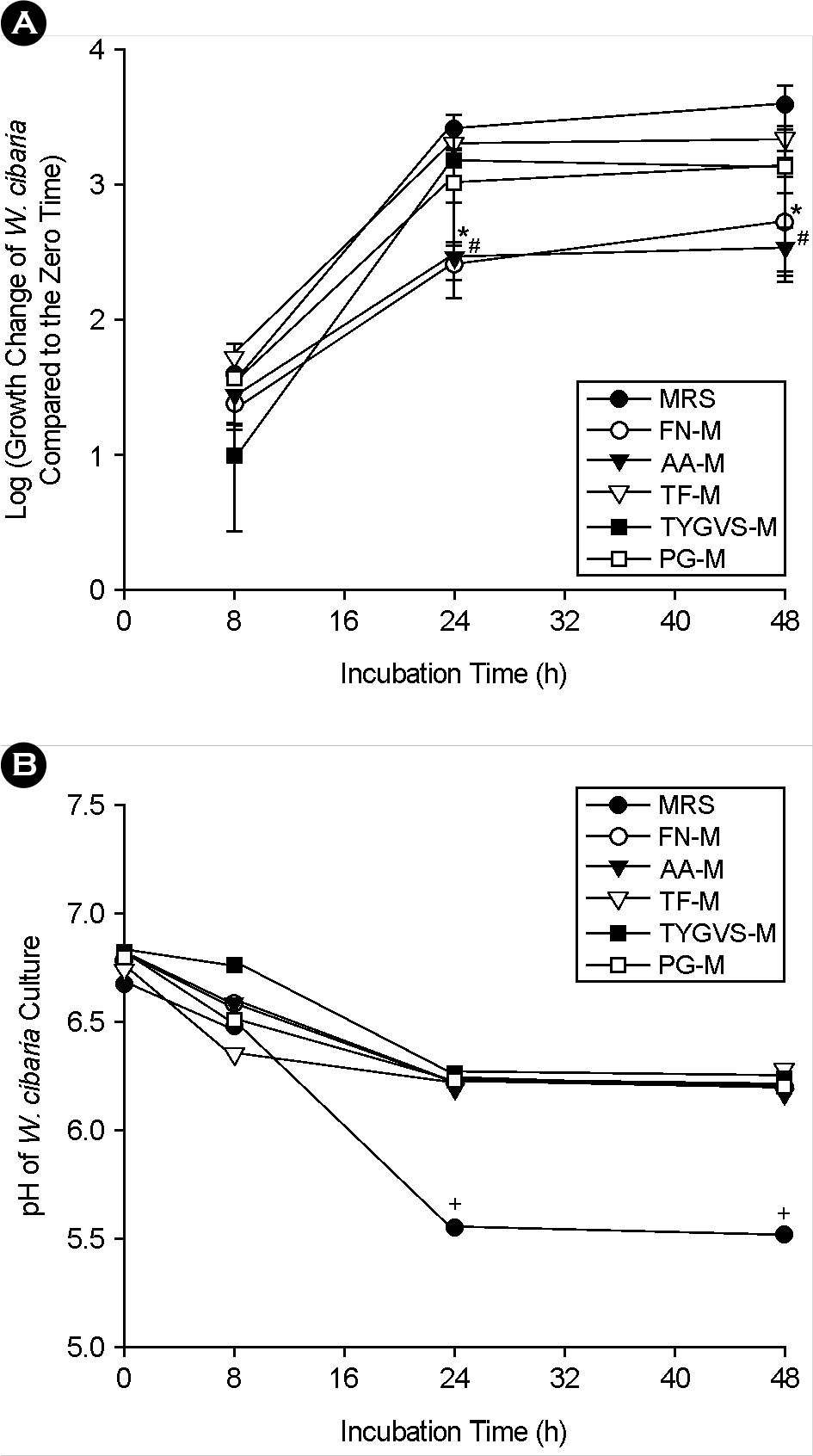 | Figure 4.Comparison of bacterial cell numbers (A) and pH changes (B) of W. cibaria over time under different growth media conditions by real-time PCR. The growth change of W. cibaria compared to the zero time (0 h) was shown as log scale. FN-M, a mixture of equal volume of F. nucleatum growth medium and MRS; AA-M, a mixture of equal volume of A. actinomycetemcomitans growth medium and MRS; TF-M, a mixture of equal volume of T. forsythia growth medium and MRS; TYGVS-M, a mixture of equal volume of T. denticola growth medium and MRS; PG-M, a mixture of equal volume of P. gingivalis growth medium and MRS. ∗p < 0.05, culture in FN-M versus culture in MRS; #p < 0.05, culture in AA-M versus culture in MRS. +p < 0.05, pH at over time versus pH at zero time. |
Table 1.
Species-specific primers and probes used for real-time PCR
Table 2.
The pH changes of periodontopathic bacterial culture over time under different growth media conditions
| Incubation time (h) | 0 | 8 | 24 | 48 |
|---|---|---|---|---|
| F. nucleatum | ||||
| FN | 6.91±0.01 | 6.93±0.03 | 6.82±0.02∗ | 6.79±0.07∗ |
| FN-M | 6.81±0.01 | 6.80±0.01 | 6.74±0.04∗ | 6.56±0.06∗ |
| A. actinomycetemcomitans | ||||
| AA | 6.91±0.01 | 6.86±0.04 | 6.91±0.05 | 6.47±0.07∗ |
| AA-M | 6.79±0.09 | 6.78±0.03 | 6.74±0.04 | 6.07±0.07∗ |
| T. forsythia | ||||
| TF | 6.82±0.06 | 6.80±0.10 | 6.83±0.13 | 6.84±0.04 |
| TF-M | 6.74±0.04 | 6.76±0.06 | 6.80±0.10 | 6.78±0.08 |
| T. denticola | ||||
| TYGVS | 7.01±0.19 | 7.01±0.19 | 7.08±0.08 | 7.08±0.08 |
| TYGVS-M | 6.86±0.02 | 6.86±0.07 | 6.84±0.04 | 6.84±0.04 |
| P. gingivalis | ||||
| PG | 6.93±0.03 | 6.97±0.06 | 7.14±0.13∗ | 7.12±0.13∗ |
| PG-M | 6.78±0.03 | 6.81±0.01 | 6.82±0.02∗ | 6.82±0.02∗ |
FN, F. nucleatum growth medium; FN-M, a mixture of equal volume of FN and MRS; AA, A. actinomycetemcomitans growth medium; AA-M, a mixture of equal volume of AA and MRS; TF, T. forsythia growth medium; TF-M, a mixture of equal volume of TF and MRS; TYGVS, T. denticola growth medium; TYGVS-M, a mixture of equal volume of TYGVS and MRS; PG, P. gingivalis growth medium; PG-M, a mixture of equal volume of PG and MRS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download