Abstract
Plasmid-mediated quinolone resistance (PMQR) genes: qnr, aac(6′)-Ib-cr, and qepA were investigated among 153 armA and 51 rmtB-positive transconjugants and their 204 clinical isolates of Enterobacteriaceae. Overall, qnrB4 and aac(6′)-Ib-cr genes were identified in 52.3% (63 K. pneumoniae, 10 E. coli, 4 E. cloacae, and 3 E. aerogenes) and 24.8% (16 K. pneumoniae, 8 E. coli, 6 S. marcescens, 4 E. cloacae, 3 C. freundii and 1 K. oxytoca) of 153 armA-positive isolates, respectively. Four isolates of K. pneumoniae and two isolates of E. coli positive for armA co-harbored both qnrB4 and aac(6′)-Ib-cr. The qepA gene was detected in 11.8% (5 E. coli and 1 K. pneumoniae) of 51 rmtB-positive clinical isolates and their transconjugants. Southern hybridization confirmed the co-localization of qepA and rmtB on a large conjugative plasmid of size between 90 to 170 kb. Inc replicon typing showed that qnrB4/6, aac(6′)-Ib-cr, and qepA genes were principally disseminated by IncFIIAs, IncL/M, and IncF plasmids, respectively. This study constitutes the first report of the three known PMQR genes among the 16S rRNA methylase producing Enterobacteriaceae isolates of human origin from Korea.
REFERENCES
1). Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother. 2003. 51:1109–17.

2). Martinez-Martinez L., Pascual A., Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet. 1998. 351:797–9.
3). Tran JH., Jacoby GA. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci USA. 2002. 99:5638–42.

4). Jacoby GA., Walsh KE., Mills DM., Walker VJ., Oh H., Robicsek A., Hooper DC. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob Agents Chemother. 2006. 50:1178–82.
5). Hata M., Suzuki M., Matsumoto M., Takahashi M., Sato K., Ibe S., Sakae K. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother. 2005. 49:801–3.
6). Nordmann P., Poirel L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother. 2005. 56:463–9.
7). Wang M., Guo Q., Xu X., Wang X., Ye X., Wu S., Hooper DC., Wang M. New plasmid-mediated quinolone resistant gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 2009. 53:1892–7.
8). Jacoby G., Cattoir V., Hooper D., Martínez-Martínez L., Nordmann P., Pascual A., Poirel L., Wang M. qnr Gene nomenclature. Antimicrob Agents Chemother. 2008. 52:2297–9.
9). Robicsek A., Strahilevitz J., Jacoby GA., Macielag M., Abbanat D., Park CH., Bush K., Hooper DC. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006. 12:83–8.

10). Ambrozic Avgustin J., Keber R., Zerjavic K., Orazem T., Grabnar M. Emergence of the quinolone resistance-mediating gene aac(6′)-Ib-cr in extended-spectrum-beta-lactamase-producing Klebsiella isolates collected in Slovenia between 2000 and 2005. Antimicrob Agents Chemother. 2007. 51:4171–3.
11). Park CH., Robicsek A., Jacoby GA., Sahm D., Hooper DC. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob Agents Chemother. 2006. 50:3953–5.
12). Wang M., Tran JH., Jacoby GA., Zhang Y., Wang F., Hooper DC. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob Agents Chemother. 2003. 47:2242–8.
13). Yamane K., Wachino J., Suzuki S., Kimura K., Shibata N., Kato H., Shibayama K., Konda T., Arakawa Y. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 2007. 51:3354–60.
14). Perichon B., Courvalin P., Galimand M. Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob Agents Chemother. 2007. 51:2464–9.
15). Cattoir V., Poirel L., Nordmann P. Plasmid-mediated quinolone resistance pump QepA2 in an Escherichia coli isolate from France. Antimicrob Agents Chemother. 2008. 52:3801–4.
16). Doi Y., Yokoyama K., Yamane K., Wachino J., Shibata N., Yagi T., Shibayama K., Kato H., Arakawa Y. Plasmid-mediated 16S rRNA methylase in Serratia marcescens conferring high-level resistance to aminoglycosides. Antimicrob Agents Chemother. 2004. 48:491–6.
17). Galimand M., Courvalin P., Lambert T. Plasmid-mediated high-level resistance to aminoglycosides in Entero-bacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother. 2003. 47:2565–71.
18). Wachino J., Yamane K., Shibayama K., Kurokawa H., Shibata N., Suzuki S., Doi Y., Kimura K., Ike Y., Arakawa Y. Novel plasmid-mediated 16S rRNA methylase, RmtC, found in a Proteus mirabilis isolate demonstrating extraordinary high-level resistance against various aminoglycosides. Antimicrob Agents Chemother. 2006. 50:178–84.
19). Doi Y., de Oliveira Garcia D., Adams J., Paterson DL. Coproduction of novel 16S rRNA methylase RmtD and metallo-beta-lactamase SPM-1 in a panresistant Pseudomonas aeruginosa isolate from Brazil. Anti-microb Agents Chemother. 2007. 51:852–6.
20). Kang HY., Kim KY., Kim J., Lee JC., Lee YC., Cho DT., Seol SY. Distribution of conjugative-plasmid-mediated 16S rRNA methylase genes among amikacin-resistant Enterobacteriaceae isolates collected in 1995 to 1998 and 2001 to 2006 at a university hospital in South Korea and identification of conjugative plasmids mediating dissemination of 16S rRNA methylase. J Clin Microbiol. 2008. 46:700–6.
21). Robicsek A., Strahilevitz J., Sahm DF., Jacoby GA., Hooper DC. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother. 2006. 50:2872–4.
22). Tamang MD., Seol SY., Oh JY., Kang HY., Lee JC., Lee YC., Cho DT., Kim J. Plasmid-mediated quinolone resistance determinants qnrA, qnrB, and qnrS among clinical isolates of Enterobacteriaceae in a Korean hospital. Antimicrob Agents Chemother. 2008. 52:4159–62.
23). Shi WF., Jiang JP., Mi ZH. Relationship between antimicrobial resistance and aminoglycoside-modifying enzyme gene expressions in Acinetobacter baumannii. Chin Med J. 2005. 118:141–5.
24). Carattoli A., Bertini A., Villa L., Falbo V., Hopkins KL., Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005. 63:219–28.

25). Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 16th Informational Supplement. M100-S16. Wayne PA: CLSI. 2006.
26). Sambrook J., Russell DW. Preparation of plasmid DNA by alkaline lysis with SDS: minipreparation. In Molecular Cloning: A laboratory manual. 3rd ed.New York: Cold spring Harbour Laboratory Press;2001. p. 1–32.
27). Ma J., Zeng Z., Chen Z., Xu X., Wang X., Deng Y., Lü D., Huang L., Zhang Y., Liu J., Wang M. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob Agents Chemother. 2009. 53:519–24.
28). Yamane K., Wachino J., Suzuki S., Arakawa Y. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob Agents Chemother. 2008. 52:1564–6.
29). Liu JH., Deng YT., Zeng ZL., Gao JH., Chen L., Arakawa Y., Chen ZL. Coprevalence of plasmid-mediated quinolone resistance determinants QepA, Qnr, and AAC(6′)-Ib-cr among 16S rRNA methylase RmtB-producing Escherichia coli isolates from pigs. Antimicrob Agents Chemother. 2008. 52:2992–3.
30). Kim HB., Park CH., Kim CJ., Kim EC., Jacoby GA., Hooper DC. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob Agents Chemother. 2009. 53:639–45.

31). Park YJ., Yu JK., Kim SI., Lee K., Arakawa Y. Accumulation of plasmid-mediated fluoroquinolone resistance genes, qepA and qnrS1, in Enterobacter aerogenes co-producing RmtB and class A β-lactamase LAP-1. Ann Clin Lab Sci. 2009. 39:55–9.
32). Cavaco LM., Hasman H., Xia S., Aarestrup FM. QnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans of human origin. Antimicrob Agents Chemother. 2009. 53:603–8.
Figure 1.
Gel-electrophoresis of plasmid DNAs extracted from transconjugants positive for qepA and rmtB (A) RFLP of Plasmid DNAs extracted from transconjugants carrying qepA and rmtB after digestion with EcoRI (B) and ApaI (C). Southern hybridizations with qepA probe (D) and with rmtB probe (E). Lanes: M, BAC-tracker™ supercoiled marker; M2, λ HindIII marker 2; 1, AKp5501JR; 2, AKp5681JR; 3, AKp5781JR; and 4, AKp5931JR.
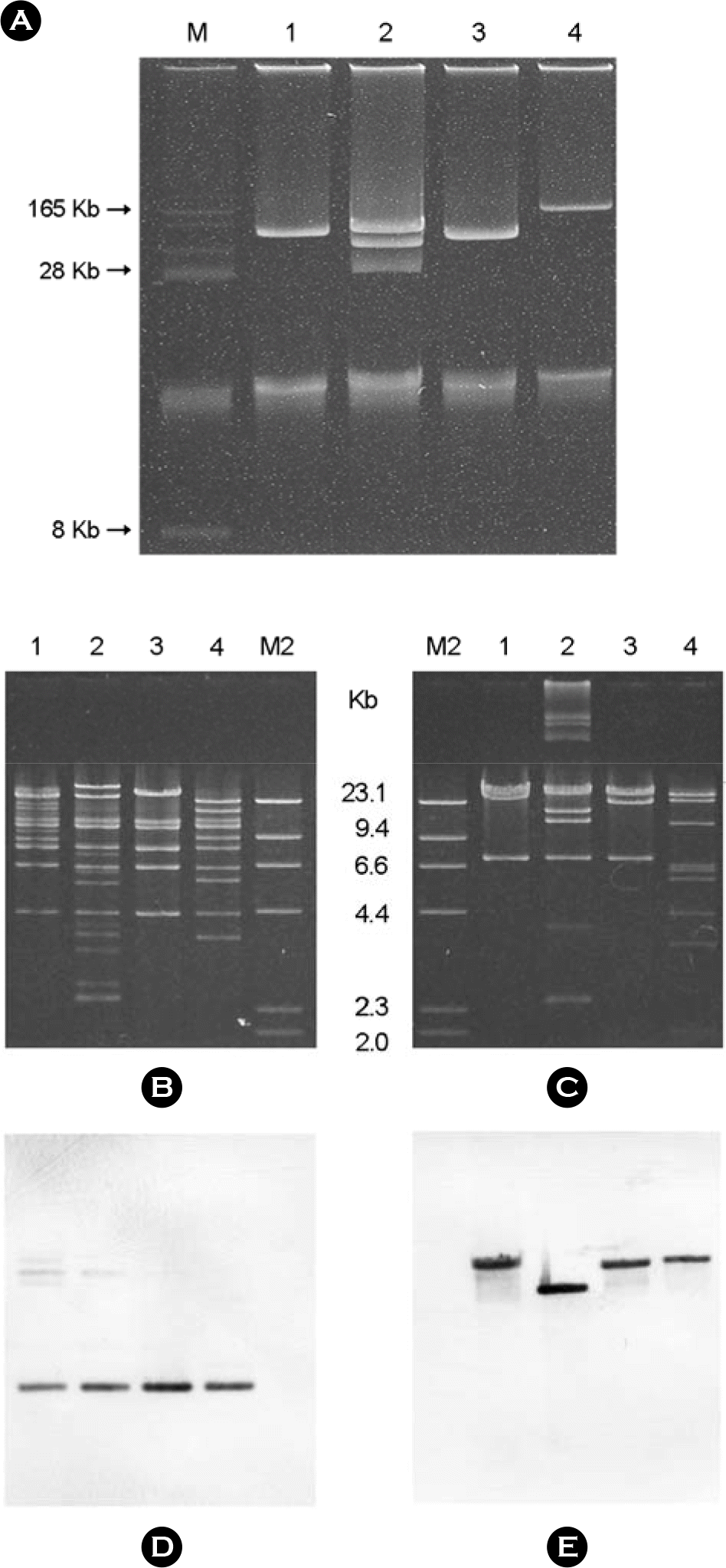
Figure 2.
Dendrogram generated by Gel Compar II showing the genomic relatedness of clinical isolates Enterobacteriaceae carrying armA and aac(6′)-Ib-cr (A) and isolates carrying qepA and rmtB (B) determined by PFGE.
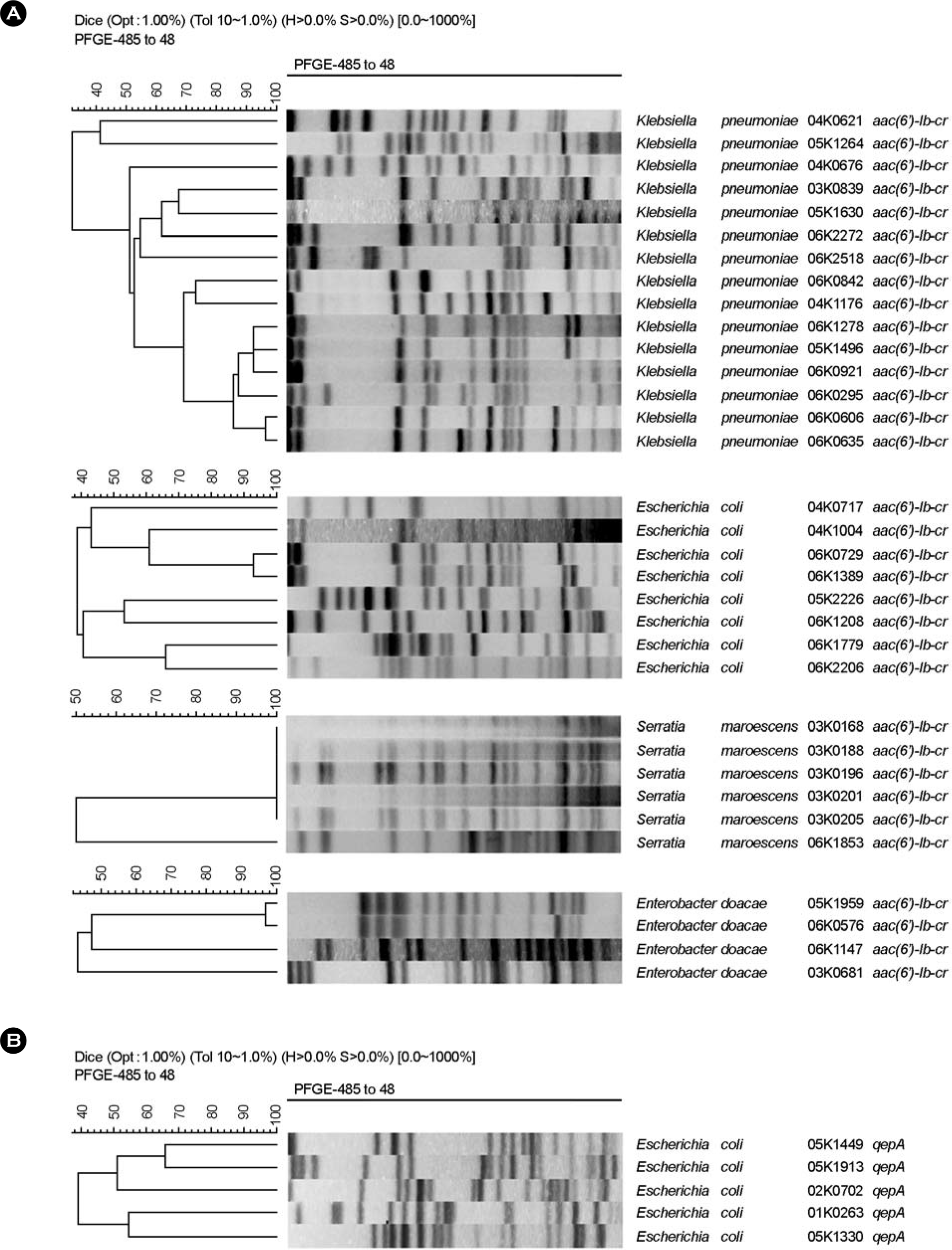
Table 1.
Distribution of plasmid-mediated qnr, aac(6′)-Ib-cr, and qepA genes among the clinical isolates of Enterobacteriaceae carrying armA or rmtB genes
Table 2.
Inc/replicon types of conjugative plasmids carrying plasmid-mediated quinolone resistance genes and armA or rmtB genes
| Plasmid-mediated quinolone resistance genes' | No. of transconjugants carrying plasmids having indicated genes and Inc plasmid | Total | |||||
|---|---|---|---|---|---|---|---|
| armA (n=153) | rmtB (n = 51) | ||||||
| A/C (n = 7) | A/C, I1-Iγ (n = 2) | FIIAs (n = 20) | L/M (n = 37) | NI a (n = 75) | F (n = 6) | ||
| qnrB4 | 15 | 61 | 76 | ||||
| qnrB6 | 1 | 1 | |||||
| Aac(6′)-Ib-cr | 2 | 1 | 32 | 35 | |||
| qepA | 6 | 6 | |||||
| Total | 2 | 1 | 16 | 32 | 61 | 6 | 118 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download