Abstract
Xenotransplantation using porcine organs could potentially associate with the risk of pathogenic infections, because human tropic porcine endogenous retrovirus (PERV) particles could be released from pig cells or organs. While there is no evidence of PERV transmission to human, safety issues become a paramount concern. For the prevention of this transmission, specific immunological tools must be provided for PERV transmission detection. In this study we described the expression of PERV envelope proteins and the production of a specific antibody against PERV envelope (Env) glycoprotein. The nucleotide sequence harboring the partial region of glycoprotein 70 was cloned into the pET vector and envelope protein was expressed in E. coli. Approximately 42 kDa recombinant Env protein (PERV Env-aa357) was purified by the Ni-affinity column. For antibody production, mice were immunized with the recombinant PERV Env-aa357. The generated anti-serum was tested using Western blot and immunocytochemical assay. We found that anti-PERV Env serum displayed the specificity against the PERV Envs (PERV-A and PERV-B) expressed not only in E. coli but also in mammalian cells, and PERV particles within the porcine cell lines (PK 15 and PK-1). Taken together, PERV antibody could be useful for detecting PERV infection or xenotransplantation transmission.
REFERENCES
2). Patience C., Takeuchi Y., Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997. 3:282–6.

3). Ritzhaupt A., Van Der Laan LJ., Salomon DR., Wilson CA. Porcine endogenous retrovirus infects but does not replicate in nonhuman primate primary cells and cell lines. J Virol. 2002. 76:11312–20.

4). Wilson CA., Wong S., Muller J., Davidson CE., Rose TM., Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998. 72:3082–7.

5). Blusch JH., Patience C., Martin U. Pig endogenous retroviruses and xenotransplantation. Xenotransplantation. 2002. 9:242–51.

6). Galbraith DN., Kelly HT., Dyke A., Reid G., Haworth C., Beekman J., Shepherd A., Smith KT. Design and validation of immunological tests for the detection of porcine endogenous retrovirus in biological materials. J Virol Methods. 2000. 90:115–24.

7). Akiyoshi DE., Denaro M., Zhu H., Greenstein JL., Banerjee P., Fishman JA. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol. 1998. 72:4503–7.

8). Le Tissier P., Stoye JP., Takeuchi Y., Patience C., Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997. 389:681–2.

9). Martin U., Kiessig V., Blusch JH., Haverich A., von der Helm K., Herden T., Steinhoff G. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet. 1998. 352:692–4.

10). Martin U., Winkler ME., Id M., Radeke H., Arseniev L., Takeuchi Y., Simon AR., Patience C., Haverich A., Steinhoff G. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation. 2000. 7:138–42.

11). Specke V., Tacke SJ., Boller K., Schwendemann J., Denner J. Porcine endogenous retroviruses: in vitro host range and attempts to establish small animal models. J Gen Virol. 2001. 82:837–44.
12). Takeuchi Y., Patience C., Magre S., Weiss RA., Banerjee PT., Le Tissier P., Stoye JP. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998. 72:9986–91.

13). Bartosch B., Stefanidis D., Myers R., Weiss R., Patience C., Takeuchi Y. Evidence and consequence of porcine endogenous retrovirus recombination. J Virol. 2004. 78:13880–90.

14). Harrison I., Takeuchi Y., Bartosch B., Stoye JP. Determinants of high titer in recombinant porcine endogenous retroviruses. J Virol. 2004. 78:13871–9.

15). Scobie L., Taylor S., Wood JC., Suling KM., Quinn G., Meikle S., Patience C., Schuurman HJ., Onions DE. Absence of replication-competent human-tropic porcine endogenous retroviruses in the germ line DNA of inbred miniature Swine. J Virol. 2004. 78:2502–9.

16). Pinter A., Kopelman R., Li Z., Kayman SC., Sanders DA. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J Virol. 1997. 71:8073–7.

17). Best S., Le Tissier PR., Stoye JP. Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol. 1997. 5:313–8.

18). Gemeniano M., Mpanju O., Salomon DR., Eiden MV., Wilson CA. The infectivity and host range of the ecotropic porcine endogenous retrovirus, PERV-C, is modulated by residues in the C-terminal region of its surface envelope protein. Virology. 2006. 346:108–17.

19). Hopp TP., Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA. 1981. 78:3824–8.

20). Lee D., Lee J., Uhm SJ., Lee YS., Park MJ., Park HY., Kwon M., Lee HT., Kim YB. Molecular characterization of the porcine endogenous retrovirus subclass A and B envelope gene from pigs. Transplant Proc. 2006. 38:3066–9.

21). Fischer N., Krach U., Niebert M., Tonjes RR. Detection of porcine endogenous retrovirus (PERV) using highly specific antisera against Gag and Env. Virology. 2003. 311:222–8.

22). Krach U., Fischer N., Czauderna F., Kurth R., Tonjes RR. Generation and testing of a highly specific anti-serum directed against porcine endogenous retrovirus nucleocapsid. Xenotransplantation. 2000. 7:221–9.

23). Tacke SJ., Bodusch K., Berg A., Denner J. Sensitive and specific immunological detection methods for porcine endogenous retroviruses applicable to experimental and clinical xenotransplantation. Xenotransplantation. 2001. 8:125–35.
Figure 1.
Expression of PERV envelope gp70 and Env-aa357. (A) Hydrophilicity of full length of PERV gp70 isolated from pig (DQ011706). The graph was drawn by Hoop-Woods method. The gray circle represents the hydrophilic C-terminal region of PERV Env-aa357. (B) SDS-PAGE (upper panel) and Western blot analysis (lower panel) of expressed PERV Envs. The expressed PERV Envs from E. coli were analyzed and stained with Coomassie blue (upper panel). Expressed recombinant proteins were confirmed with an anti-His antibody (lower panel). M, protein marker; lane 1, BL21 (DE3) pLysS; lane 2, pET-gp70 transformed cell lysate before induction; lane 3, pET-gp70 transformed cell lysate after induction; lane 4, pET-aa357 transformed cell lysate before induction; lane 5, pET-aa357 transformed cell lysate after induction. The arrow indicates molecular mass of aa357 protein, approximately 42 kDa.
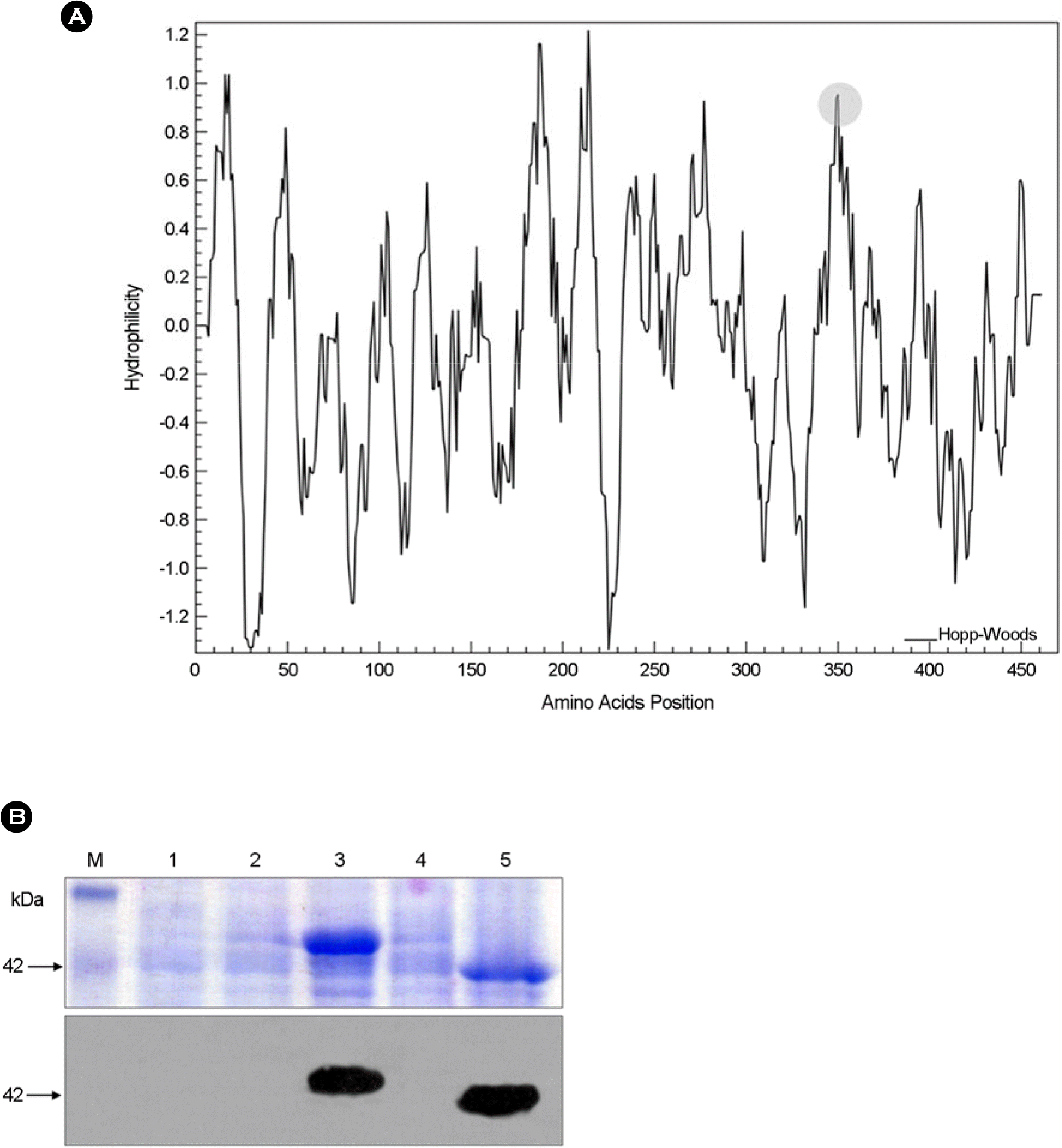
Figure 2.
Purification of the recombinant PERV Env-aa357. Affinity purified Env protein (≈ 50 μg) was analyzed on a 10% SDS-PAGE gel (A) and stained with Coomassie blue and confirmed with the mouse anti-PERV antibody (B). Lane 1, E. coli BL21 (DE3) pLysS; lane 2, pET-aa357 transformed cell lysate before induction; lane 3, pET-aa357 transformed cell lysate after induction; lane 4, purified PERV Env-aa357.
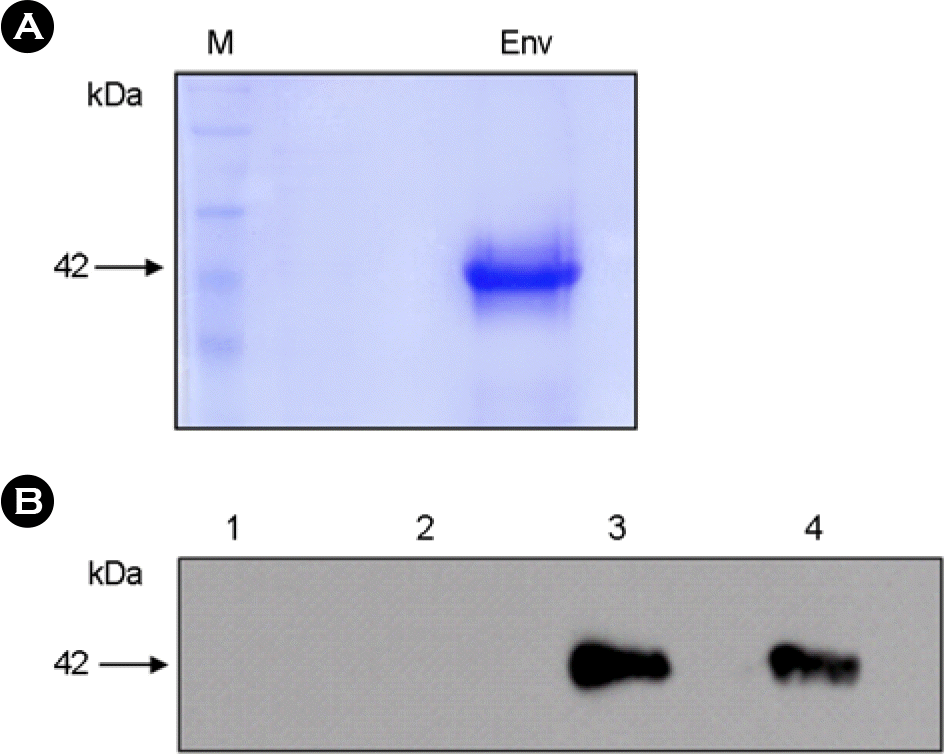
Figure 3.
Western blot analysis of the constructed anti-PERV antibody with PERV gp70 envelope proteins expressed in mammalian cells. The two types of PERV-A and -B envelope glycoprotein constructs were expressed in the 293T cells and those cell proteins were analyzed with the constructed anti-PERV antibody. PERV proteins originated from 293T cells were expressed by a previous method using vaccinia expression system.
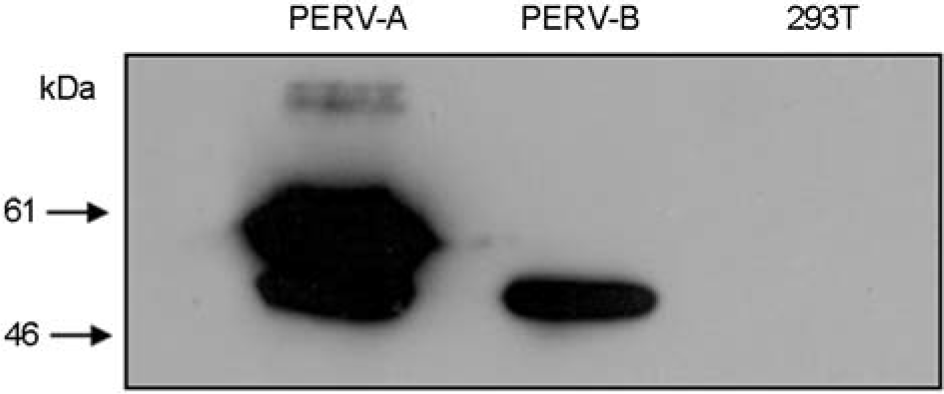
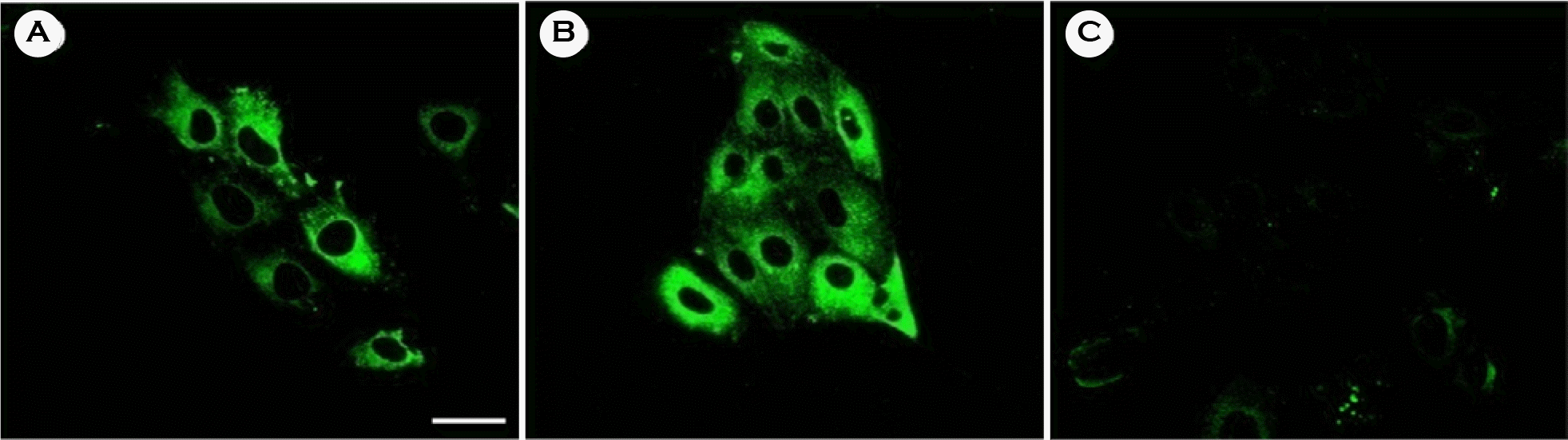
Figure 4.
Immunocytochemical staining of the PERV producing porcine cells with constructed anti-PERV antibody. Porcine cells were stained with the constructed anti-PERV antibody and FITC conjugated anti-mouse secondary antibody, and visualized by confocal laser microscopy. FITC staining was measured at 488 nm and non-specific fluorescence measured at 543 nm was subtracted. Indirect immuno-fluorescence was visualized with an Olympus confocal microscope FV1000 at a magnification of 60 X. PK15 cell (A), PK-1 cell (B), and HeLa cell (C). The scale bar represents 30 μm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download