Abstract
Biofilms are microbial communities that form on a surface and are surrounded by extracellular polymeric substances. Candida biofilms are a cause of infections associated with medical devices. In the present study, an attempt was made to evaluate a significance of biofilm formation ability (BF) in virulence of C. albicans. C. albicans of 98 isolates, 24 commensal strains obtained from the oral cavities of healthy volunteers, 29 from blood culture, 25 from urine culture, and 20 from vaginal candidiasis, were assayed for BF, an ability to adhere to epithelial cells (ADH), cell surface hydrophobicity (CSH), and germ tube forming rate (GT). The relationships of BF with CSH, ADH, and GT were statistically examined. A positive correlation between BF and ADH was obtained, but the correlation (r=0.326) was relatively low. To assess BF as a factor contributing for candidiasis, mice lethality test was performed. The 10 isolates with the highest BF (mean survival rate, 24%) allow to kill mice more than those with the 10 lowest BF (mean survival rate, 47%). In addition, clinical strains isolated from blood culture, urine culture, and vaginal candidiasis showed higher BF than oral commensal strains. These results suggest BF may represent a virulent characteristic of C. albicans.
Go to : 
REFERENCES
1). Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE; Infectious Diseases Society of America. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004. 38:161–89.
2). Hurley R., de Louvois J., Mulhall A. Yeasts as human and animal pathogens. Rose AH, Harrison JS, editors. editors.The yeasts. 2nd ed.London: Academic Press INC;1987. p. 212 39.
3). Calderone R., Gow NAR. Host recognition by Candida species. Calderone RA, editor. editor,. Candida and candidiasis. Washington D.C.: ASM Press;2002. p. 67–86.
4). Hostetter MK. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin Microbiol Rev. 1994. 7:29–42.
5). Sundstrom P. Adhesion in Candida spp. Cell Microbiol. 2002. 4:461–9.
6). Hube B., Naglik J. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology. 2001. 147:1997–2005.
7). Hube B., Naglik JR. Extracellular hydrolases. Calderone RA, editor. editor,. Candida and candidiasis. Washington D.C.: ASM Press;2002. p. 107–22.
8). Brown AJ., Gow NA. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999. 7:333–8.
9). Gow NA., Brown AJ., Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002. 5:366–71.

10). Soll DR. Gene regulation during high-frequency switching in Candida albicans. Microbiology. 1997. 143:279–88.
11). Soll DR. Phenotypic switching. Calderone RA, editor. editor,. Candida and candidiasis. Washington D.C.: ASM Press;2002. p. 123–42.
12). Kojic EM., Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004. 17:255–67.
13). Ramage G., Martínez JP., Lípez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006. 6:979–86.
14). Seneviratne CJ., Jin L., Samaranayake LP. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008. 14:582–90.
15). Ramage G., Vandewalle K., Wickes BL., Lopez-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001. 18:163–70.
16). Baillie GS., Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000. 46:397–403.
17). Andes D., Nett J., Oschel P., Albrecht R., Marchillo K., Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004. 72:6023–31.
18). Rosenberg M. Microbial adhesion to hydrocarbons: twenty-five years of doing MATH. FEMS Microbiol Lett. 2006. 262:129–34.

19). Chaffin WL., López-Ribot JL., Casanova M., Gozalbo D., Martínez JP. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998. 62:130–80.
20). Li X., Yan Z., Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003. 149:353–62.
Go to : 
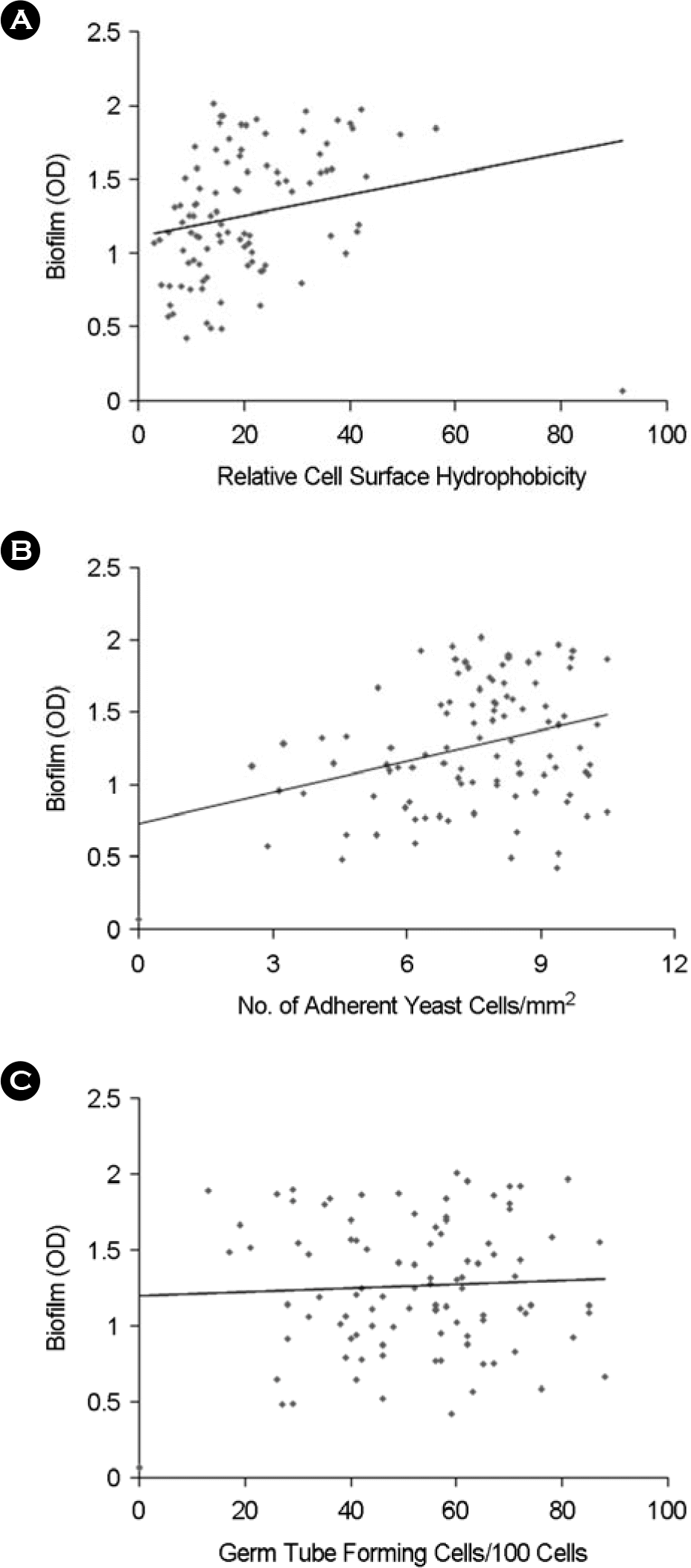 | Figure 1.Correlation between the ability of biofilm formation (BF) and putative virulence factors of C. albicans. A. Correlation between BF and relative cell surface hydrophobicity: r = 0.219, p = 0.07; B. Correlation between BF and the ability of adherence to HeLa cells: r = 0.326, p < 0.01; C. Correlation between BF and germ tube forming rate: r = 0.045, p = 0.61. |
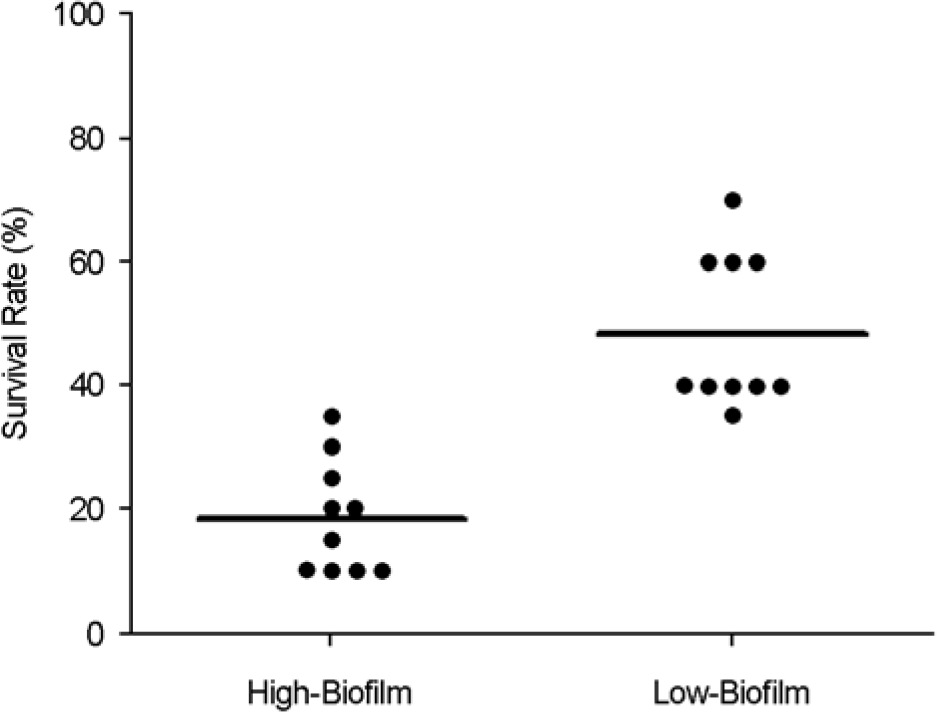 | Figure 2.Effect of C. albicans BF on lethality in mice. The 10 isolates with the highest BF and the 10 isolates with the lowest BF were tested. Twenty mice per Candida isolate were injected intravenously via tail vein with a total of 0.2 ml normal saline containing 5 × 105 cells. High BF-C. albicans (mean survival day: 25 days, mean survival rate: 24%) allow to kill mice more than low BF-C. albicans (mean survival day: 40 days, mean survival rate: 47%) (p < 0.01). • represents a survival rate of one isolate tested. |
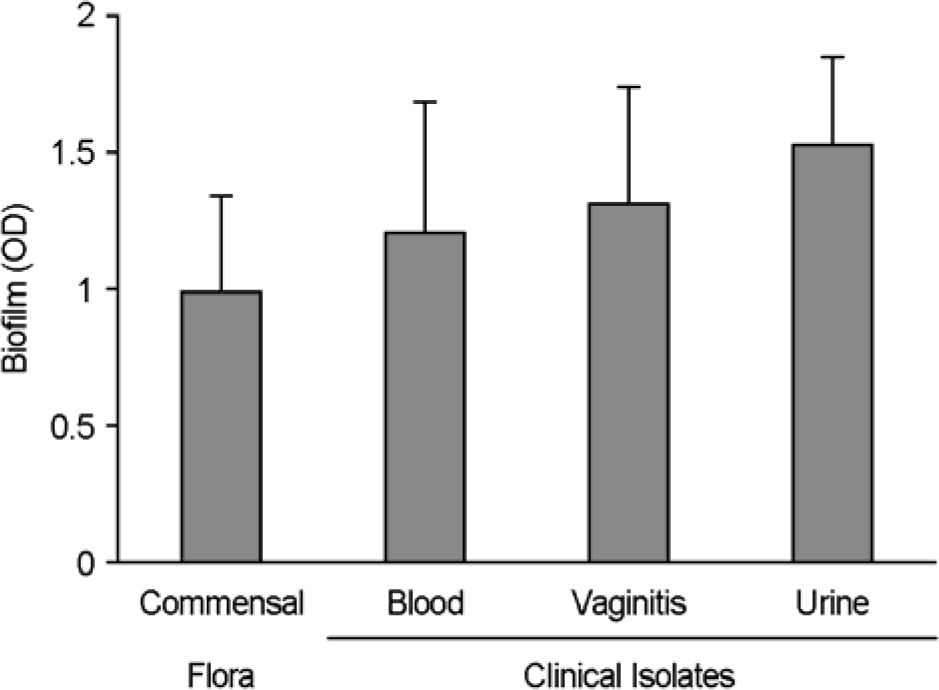 | Figure 3.Difference in BF among different C. albicans isolates. Commensal strains (n = 24) isolated from the oral cavities of healthy carrier and clinical isolates from blood culture (n=29), urine culture (n = 25), and vaginal candidiasis (n = 20) were assayed for BF. XTT activities were measured colorimetrically at 490 nm with microtiter plate reader. Each bar represents the mean from three independent experiments (p < 0.01). Error bars mean standard deviations. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download