Abstract
Ginsan, a botanic polysaccharide extracted from Panax ginseng, has recently been reported to modulate mucosal immune response. In this study, we investigated the protective effect of Ginsan against fatal Vibrio vulnificus mucosal infection. A lethal dose of V. vulnificus (1.0 × 106 CFU/mouse) was nasally inoculated to mice. The bacterial count in the nasal associated lymphoid tissue (NALT) of the mouse was significantly reduced in the Ginsan-treated group. The Ginsan-treated group showed improved survival compared to the control group (100% vs 18%). To elucidate the effect of Ginsan on modulating host immune response, cytokine mRNA expressions involved in mediating inflammation were determined by semiquantitative RT-PCR in the NALTs of the infected mice. Most of the cytokine mRNAs were similarly expressed as the control group. However, COX-1 mRNA expression level was higher in Ginsan-treated group compared to the control group. The protective effect of Ginsan was antagonized by treating with a specific COX-1 inhibitor, SC-560. Thus, these data suggest that the protective effect of Ginsan against V. vulnificus infection is partly mediated by modulating COX-1 expression.
REFERENCES
1). Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000. 13:523–33.

2). Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002. 60:258–74.
3). Xiang YZ., Shang HC., Gao XM., Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008. 22:851–8.

4). Robbers JE., Tyler VE. Tyler's Herbs of choice: the therapeutic use of phytomedicinals. New York: Haworth Herbal Press. 1999.
5). Song JY., Han SK., Son EH., Pyo SN., Yun YS., Yi SY. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002. 2:857–65.

6). Lim DS., Bae KG., Jung IS., Kim CH., Yun YS., Song JY. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J Infect. 2002. 45:32–8.
7). Dann SM., Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007. 23:115–20.

8). Wallace JL., Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000. 119:512–20.

9). Playford RJ., Ghosh S. Cytokines and growth factor modulators in intestinal inflammation and repair. J Pathol. 2005. 205:417–25.

10). Mármol F., Sánchez J., López D., Martínez N., Mitjavila MT., Puig-Parellada P. Oxidative stress, nitric oxide and prostaglandin E2 levels in the gastrointestinal tract of aging rats. J Pharm Pharmacol. 2009. 61:201–6.

11). Robert A., Aristoff PA., Wendling MG., Kimball FA., Miller WL Jr., Gorman RR. Cytoprotective and anti-secretory properties of a non-diarrheogenic and non-uterotonic prostacyclin analog: U-68,215. Prostaglandins. 1985. 30:619–49.
12). Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997. 112:1000–16.

13). Lim YH., Na HS., Yun YS., Choi IS., Oh JS., Rhee JH., Cho BH., Lee HC. Suppressive effects of Ginsan on the development of allergic reaction in murine asthmatic model. Int Arch Allergy Immunol. 2009. 150:32–42.

14). Linkous DA., Oliver JD. Pathogenesis of Vibrio vulnificus. FEMS Microbiol Lett. 1999. 174:207–14.
15). Han SK., Song JY., Yun YS., Yi SY. Ginsan improved Th1 immune response inhibited by gamma radiation. Arch Pharm Res. 2005. 28:343–50.

16). Ahn JY., Choi IS., Shim JY., Yun EK., Yun YS., Jeong G., Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006. 36:37–45.

17). Oliver JD. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol Infect. 2005. 133:383–91.
18). Hor LI., Chang YK., Chang CC., Lei HY., Ou JT. Mechanism of high susceptibility of iron-overloaded mouse to Vibrio vulnificus infection. Microbiol Immunol. 2000. 44:871–8.
19). Park HS., Francis KP., Yu J., Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol. 2003. 171:2532–7.

20). Hyland KA., Brennan R., Olmsted SB., Rojas E., Murphy E., Wang B., Cleary PP. The early interferon response of nasal-associated lymphoid tissue to Streptococcus pyogenes infection. FEMS Immunol Med Microbiol. 2009. 55:422–31.
21). Kiyono H., Fukuyama S. NALT-versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004. 4:699–710.
22). Smith WL., Dewitt DL. Prostaglandin endoperoxide H synthases-1 and −2. Adv Immunol. 1996. 62:167–215.

23). Sales KJ., Katz AA., Howard B., Soeters RP., Millar RP., Jabbour HN. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin e receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002. 62:424–32.
Figure 1.
Effect of Ginsan on V. vulnificus intranasal infection. (A) BALB/c mice were pretreated with Ginsan (100 mg/kg) for two days, control mice with PBS. On the 3rd day, mice were nasally inoculated with 1 × 106 V. vulnificus in 10 μl PBS. Mice were killed 4 hrs after infection and the number of V. vulnificus in the NALT was determined by plating on HI agars. (∗p <0.05) (B) Mouse survival following intranasal V. vulnificus infection. Mice were observed for 7 days and live mice were counted (each group consists of 6 mice).
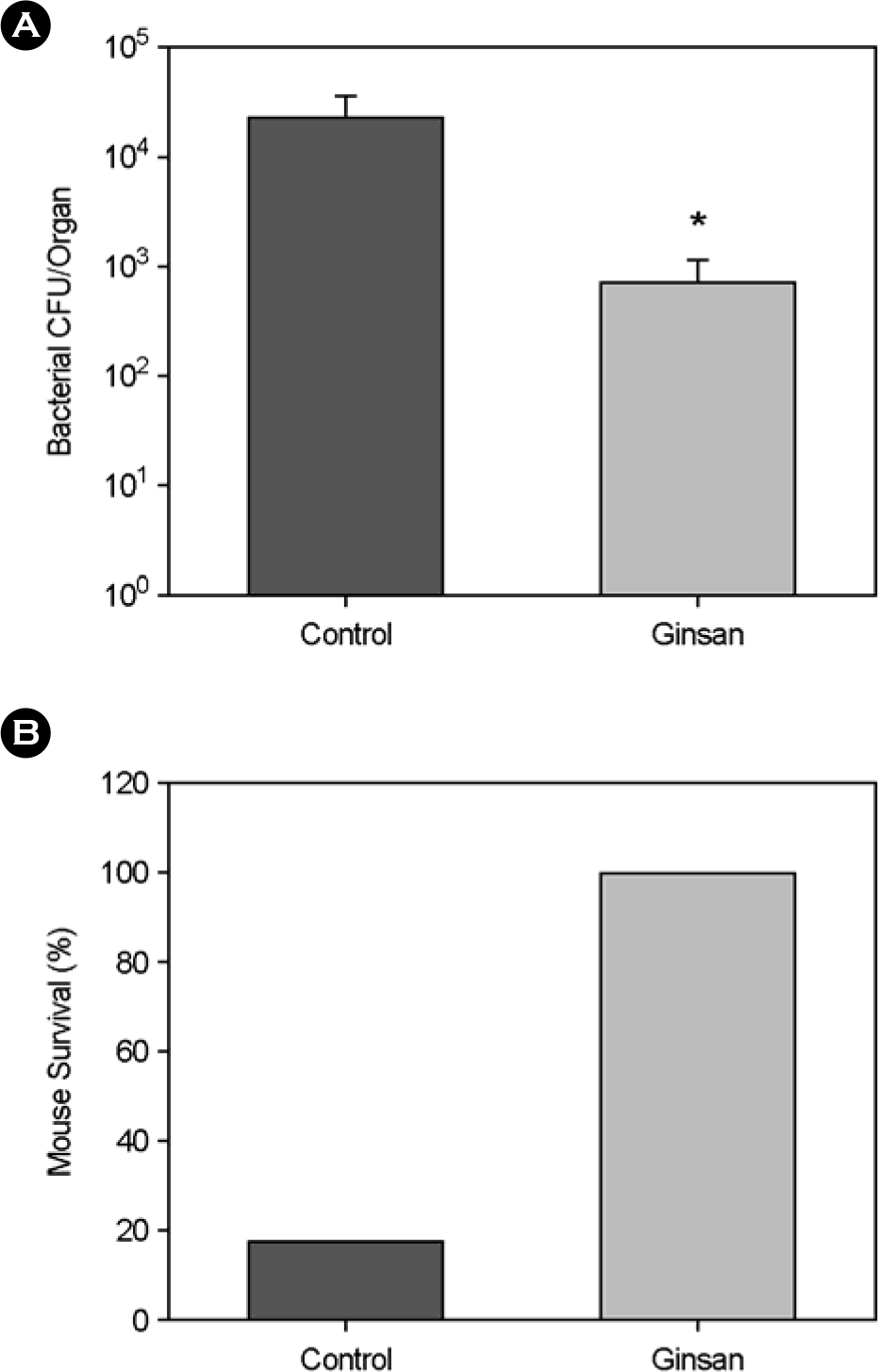
Figure 2.
Semiquantitative RT-PCR for mRNA expression of inflammatory cytokine genes. (A) BALB/c mice were treated with Ginsan (100 mg/kg) for two days, control mice with PBS. On the 3rd day, mice were nasally inoculated with 1 × 106 V. Vulnificus in 10 μl PBS. Mice were killed 2 hrs after infection and a semiquantitative RT-PCR for various inflammatory cytokine mRNA expressions was performed. (B) A semiquantitative RT-PCR for COX-1 mRNA from the NALT at 1, 2, and 4 hrs following V. vulnificus intranasal infection.
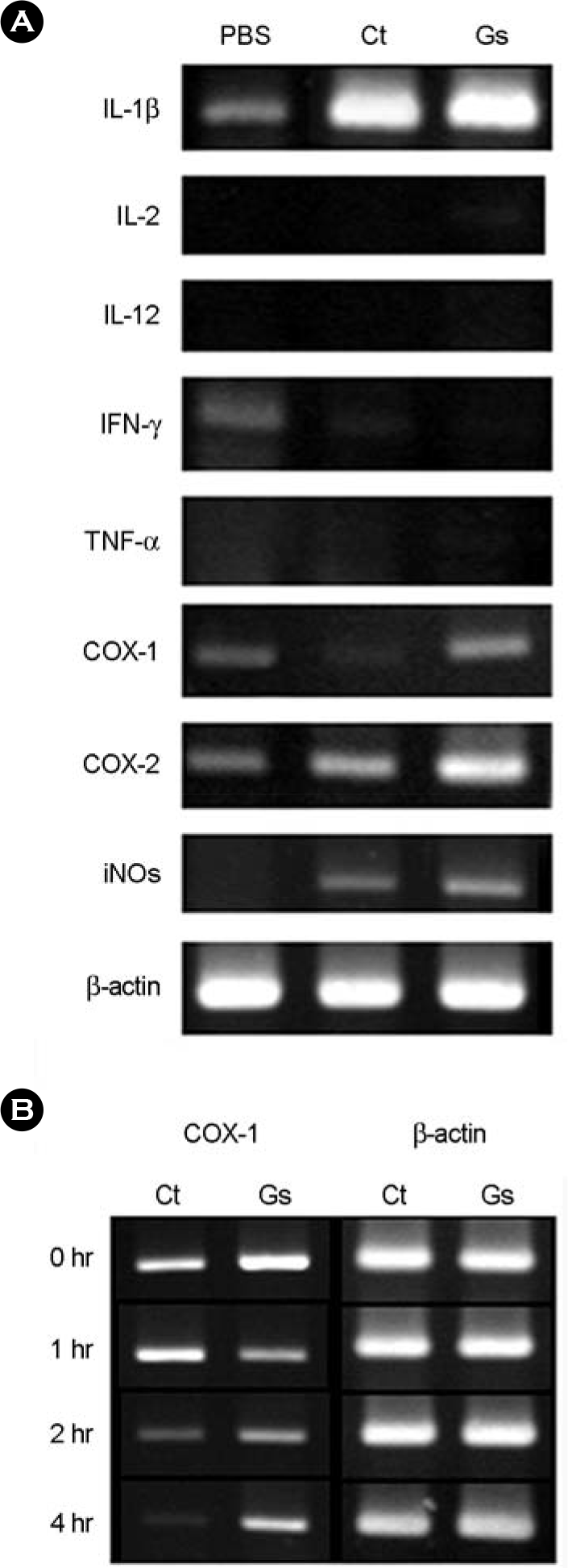
Figure 3.
Effect of Ginsan and/or SC-560 pretreatment on V. vulnificus intranasal infection. BALB/c mice were treated with Ginsan (100 mg/kg) for two days, control mice with PBS. On the 3rd day, 1 hr before bacterial challenge, SC-560 (2.5 mg/kg), a specific COX-1 inhibitor, was orally administered. And then mice were nasally inoculated with 1 × 106 V. Vulnificus in 10 μl PBS and were sacrificed 4 hrs after infection to enumerate the number of the bacteria in the NALT (∗p < 0.05: control vs Ginsan).

Table 1.
Primers used for semiquantitative RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download