Abstract
Upon contact with airway epithelial cells, mycobacteria activate several signal transduction events that are required for induction of inflammatory cytokines/chemokines. In this study, we found that Mycobacterium tuberculosis (Mtb)-induced reactive oxygen species (ROS) production is essential for the expression of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and CXC-chemokine ligand (CXCL) 8 through the activation of mitogen-activated protein kinases [MAPKs; extracellular signal-regulated kinase (ERK) 1/2 and p38 MAPK] in A549 cells representing alveolar epithelial cells. We observed that Mtb rapidly enhanced ROS production after stimulation in a toll-like receptor (TLR) 2-dependent manner. In addition, Mtb triggered ERK1/2 and p38 MAPK signaling pathways which were dependent on ROS generation in A549 cells. Moreover, Mtb stimulation significantly increased the secretion of TNF-α, IL-6, and CXCL8 over that in untreated controls. Pretreatment of A549 cells with the antioxidant, N-acetylcysteine and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor, diphenylene iodonium, substantially inhibited Mtb-induced production of TNF-α, IL-6, and CXCL8. Studies using inhibitors selective for ERK1/2 and p38 MAPK pathways showed that both pathways play an essential role in the induction of TNF-α, IL-6, and CXCL8 at transcriptional levels in A549 cells. Collectively, our findings indicate the critical role of TLR2-dependent ROS in the Mtb-induced inflammatory cytokine/chemokine production in alveolar epithelial cells through MAPK-dependent signaling pathways.
Go to : 
REFERENCES
1). Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. 1991. Bull World Health Organ. 2001. 79:71–5.
2). The World Health Organization: Tuberculosis. Fact sheet number 104. 2006.
3). Bloom BR., Small PM. The evolving relation between humans and Mycobacterium tuberculosis. N Engl J Med. 1998. 338:677–8.
4). Jo EK., Park JK., Dockrell HM. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr Opin Infect Dis. 2003. 16:205–10.

5). Jo EK., Yang CS., Choi CH., Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007. 9:1087–98.

6). Roy S., Sharma S., Sharma M., Aggarwal R., Bose M. Induction of nitric oxide release from the human alveolar epithelial cell line A549: an in vitro correlate of innate immune response to Mycobacterium tuberculosis. Immunology. 2004. 112:471–80.
7). Aslan M., Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal. 2003. 5:781–8.

8). Chandel NS., Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007. 42:165–74.

9). Poli G., Leonarduzzi G., Biasi F., Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004. 11:1163–82.

10). Lee HM., Shin DM., Kim KK., Lee JS., Paik TH., Jo EK. Roles of reactive oxygen species in CXCL8 and CCL2 expression in response to the 30-kDa antigen of Mycobacterium tuberculosis. J Clin Immunol. 2009. 29:46–56.
11). Yang CS., Shin DM., Lee HM., Son JW., Lee SJ., Akira S., Gougerot-Pocidalo MA., El-Benna J., Ichijo H., Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008. 10:741–54.

12). Yuk JM., Shin DM., Yang CS., Kim KH., An SJ., Rho J., Park JK., Jo EK. Role of apoptosis-regulating signal kinase 1 in innate immune responses by Mycobacterium bovis bacillus Calmette-Guerin. Immunol Cell Biol. 2009. 87:100–7.
13). Méndez-Samperio P., Trejo A., Pérez A. Mycobacterium bovis Bacillus Calmette-Guerin (BCG) stimulates IL-10 production via the PI3K/Akt and p38 MAPK pathways in human lung epithelial cells. Cell Immunol. 2008. 251:37–42.
14). Méndez-Samperio P., Miranda E., Trejo A. Expression and secretion of cathelicidin LL-37 in human epithelial cells after infection by Mycobacterium bovis Bacillus Calmette-Guerin. Clin Vaccine Immunol. 2008. 15:1450–5.
15). Schorey JS., Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003. 5:133–42.

16). Yang CS., Lee JS., Song CH., Hur GM., Lee SJ., Tanaka S., Akira S., Paik TH., Jo EK. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 2007. 9:382–96.
17). Song CH., Lee JS., Lee SH., Lim K., Kim HJ., Park JK., Paik TH., Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003. 23:194–201.
18). Jung SB., Yang CS., Lee JS., Shin AR., Jung SS., Son JW., Harding CV., Kim HJ., Park JK., Paik TH., Song CH., Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006. 74:2686–96.

19). Martindale JL., Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002. 192:1–15.

20). Yodoi J., Masutani H., Nakamura H. Redox regulation by the human thioredoxin system. Biofactors. 2001. 15:107–11.

21). Gribar SC., Anand RJ., Sodhi CP., Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008. 83:493–8.

22). Schmeck B., Huber S., Moog K., Zahlten J., Hocke AC., Opitz B., Hammerschmidt S., Mitchell TJ., Kracht M., Rosseau S., Suttorp N., Hippenstiel S. Pneumococci induced TLR- and Rac1-dependent NF-kappaB-recruitment to the IL-8 promoter in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006. 290:L730–7.
23). Guillot L., Medjane S., Le-Barillec K., Balloy V., Danel C., Chignard M., Si-Tahar M. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling path-ways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004. 279:2712–8.
24). Armstrong L., Medford AR., Uppington KM., Robertson J., Witherden IR., Tetley TD., Millar AB. Expression of functional toll-like receptor-2 and −4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004. 31:241–5.

25). Flynn JL., Goldstein MM., Chan J., Triebold KJ., Pfeffer K., Lowenstein CJ., Schreiber R., Mak TW., Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995. 2:561–72.
26). Mohan VP., Scanga CA., Yu K., Scott HM., Tanaka KE., Tsang E., Tsai MM., Flynn JL., Chan J. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001. 69:1847–55.

28). Flesch IE., Kaufmann SH. Stimulation of antibacterial macrophage activities by B-cell stimulatory factor 2 (interleukin-6). Infect Immun. 1990. 58:269–71.

29). VanHeyningen TK., Collins HL., Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol. 1997. 158:330–7.
30). Serbina NV., Jia T., Hohl TM., Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008. 26:421–52.

31). Lin Y., Zhang M., Barnes PF. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun. 1998. 66:1121–6.
32). Baggiolini M., Walz A., Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989. 84:1045–9.

33). Larsen CG., Anderson AO., Appella E., Oppenheim JJ., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989. 243:1464–6.

34). Leonard EJ., Skeel A., Yoshimura T., Noer K., Kutvirt S., Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990. 144:1323–30.
35). Zhang Y., Broser M., Cohen H., Bodkin M., Law K., Reibman J., Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995. 95:586–92.
36). Nibbering PH., Pos O., Stevenhagen A., Van Furth R. Interleukin-8 enhances nonoxidative intracellular killing of Mycobacterium fortuitum by human granulocytes. Infect Immun. 1993. 61:3111–6.
37). Larsen CG., Thomsen MK., Gesser B., Thomsen PD., Deleuran BW., Nowak J., Sk⊘dt V., Thomsen HK., Deleuran M., Thestrup-Pedersen K. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J Immunol. 1995. 155:2151–7.
38). Obata T., Brown GE., Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med. 2000. 28:N67–77.

39). Sun T., Dittmar K., Koduru P., Susin M., Teichberg S., Brody J. Relationship between hairy cell leukemia variant and splenic lymphoma with villous lymphocytes: presentation of a new concept. Am J Hematol. 1996. 51:282–8.

40). Kim H., Hwang JS., Woo CH., Kim EY., Kim TH., Cho KJ., Kim JH., Seo JM., Lee SS. TNF-alpha-induced up-regulation of intercellular adhesion molecule-1 is regulated by a Rac-ROS-dependent cascade in human airway epithelial cells. Exp Mol Med. 2008. 40:167–75.
41). Zhao Y., Usatyuk PV., Gorshkova IA., He D., Wang T., Moreno-Vinasco L., Geyh AS., Breysse PN., Samet JM., Spannhake EW., Garcia JG., Natarajan V. Regulation of COX-2 expression and IL-6 release by particulate matter in airway epithelial cells. Am J Respir Cell Mol Biol. 2009. 40:19–30.

42). Coburn J., Frank DW. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect Immun. 1999. 67:3151–4.
Go to : 
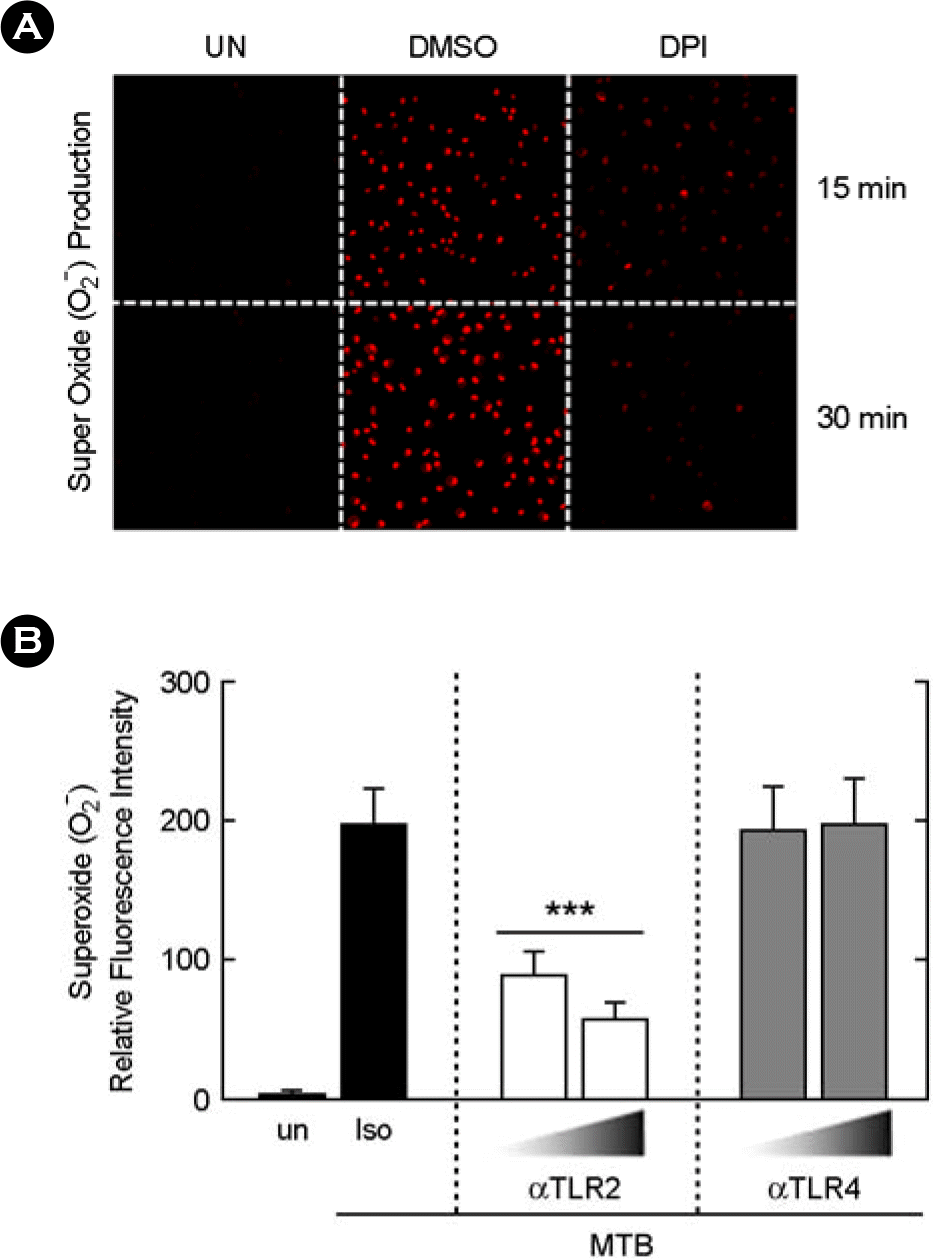 | Figure 1.Mtb-induced ROS generation is dependent on the TLR2, but not TLR4, in A549 cells. (A) The A549 cells were stimulated with Mtb (MOI = 5) for 15 to 30 min and then DHE assays were performed. Representative immunofluorescence images are shown. The data shown are representative of three experiments. (B) The experimental conditions follow the same pattern as outlined in Panel A. The A549 cells were pre-incubated with an anti-TLR2 (αTLR2), anti-TLR4 (αTLR4; both 5, 10 μg/ml) or an isotype control mAb (10 μg/ml), followed by the stimulation with Mtb (MOI = 5) for 30 min. The quantitative data for DHE (for superoxide) fluorescence shown are the mean ± SD of three experiments. Significant differences (∗∗∗, p < 0.001). UN, unstimulated; Iso, isotype control. |
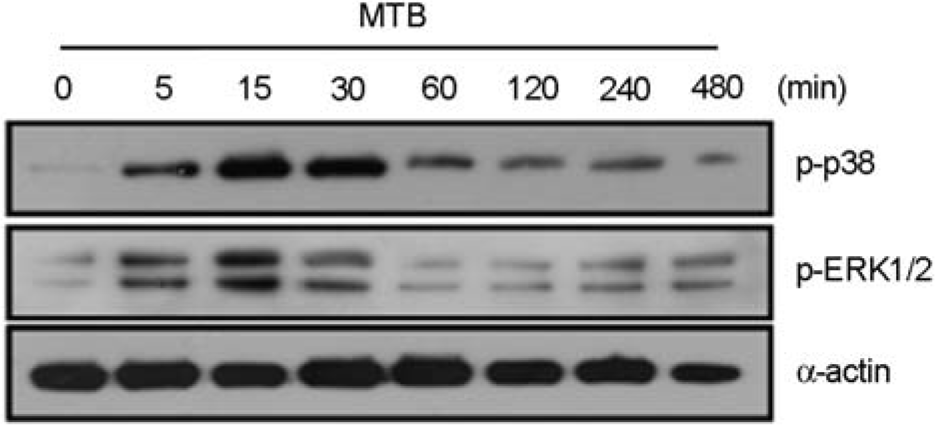 | Figure 2.Mtb rapidly induces the phosphorylation of MAPKs by A549 cells. The A549 cells were stimulated with Mtb (MOI = 5) for the indicated times (0~480 min). The cells were harvested and subjected to Western blot analysis for phosphorylated ERK1/2 and p38 MAPK. The same blots were washed and blotted for α-actin as the loading controls. Data are representative of five independent experiments with similar results. |
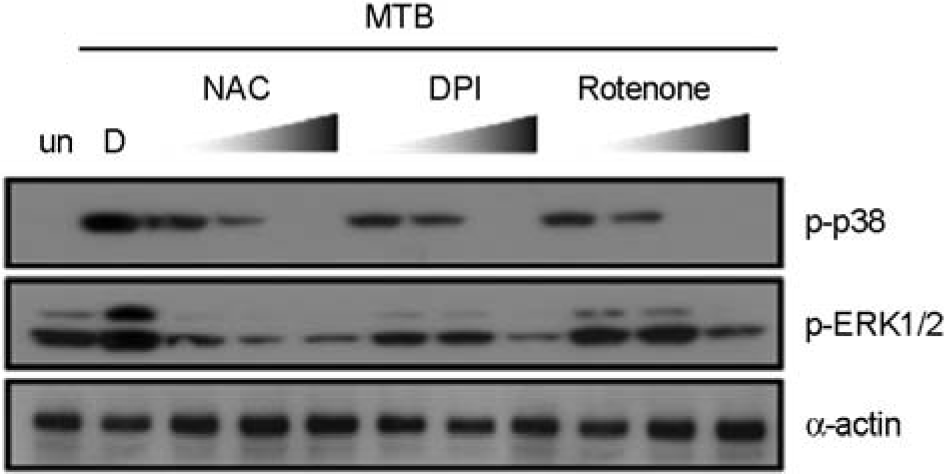 | Figure 3.Intracellular ROS production is essential for Mtb-induced MAPK activation by A549 cells. The A549 cells were pretreated with or without NAC (10, 50, 100 mM), DPI (5, 10, 20 μM) or rotenone (5, 10, 20 μM) for 45 min before stimulation with Mtb (MOI = 5). The cells were harvested after 15 min and subjected to Western blot analysis for phosphorylated ERK1/2 and p38 MAPK. The same blots were washed and blotted for α-actin as the loading controls. Data are representative of three independent experiments with similar results. un, unstimulated; D, solvent control (0.1% DMSO). |
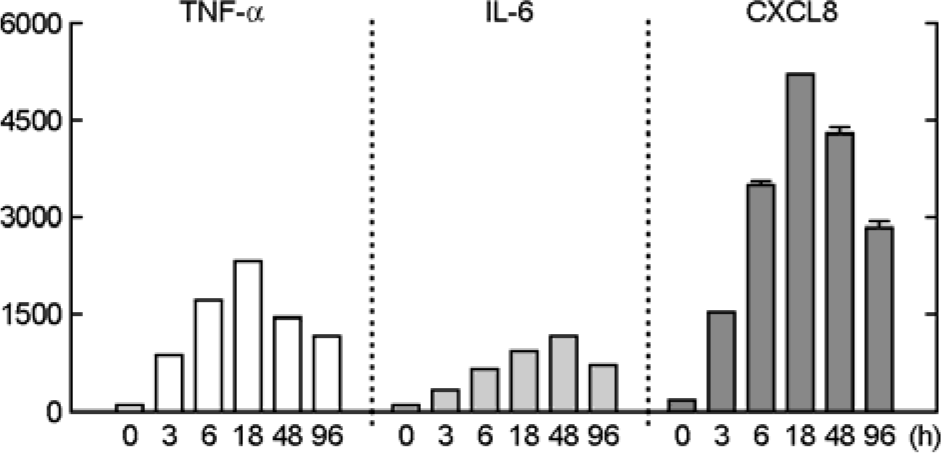 | Figure 4.Mtb induces pro-inflammatory cytokines and chemokine production by A549 cells. The A549 cells were stimulated with Mtb (MOI = 5) for the indicated times and then performed ELISA analysis (for TNF-α, IL-6 and CXCL8). Data are the mean ± SD of three experiments. |
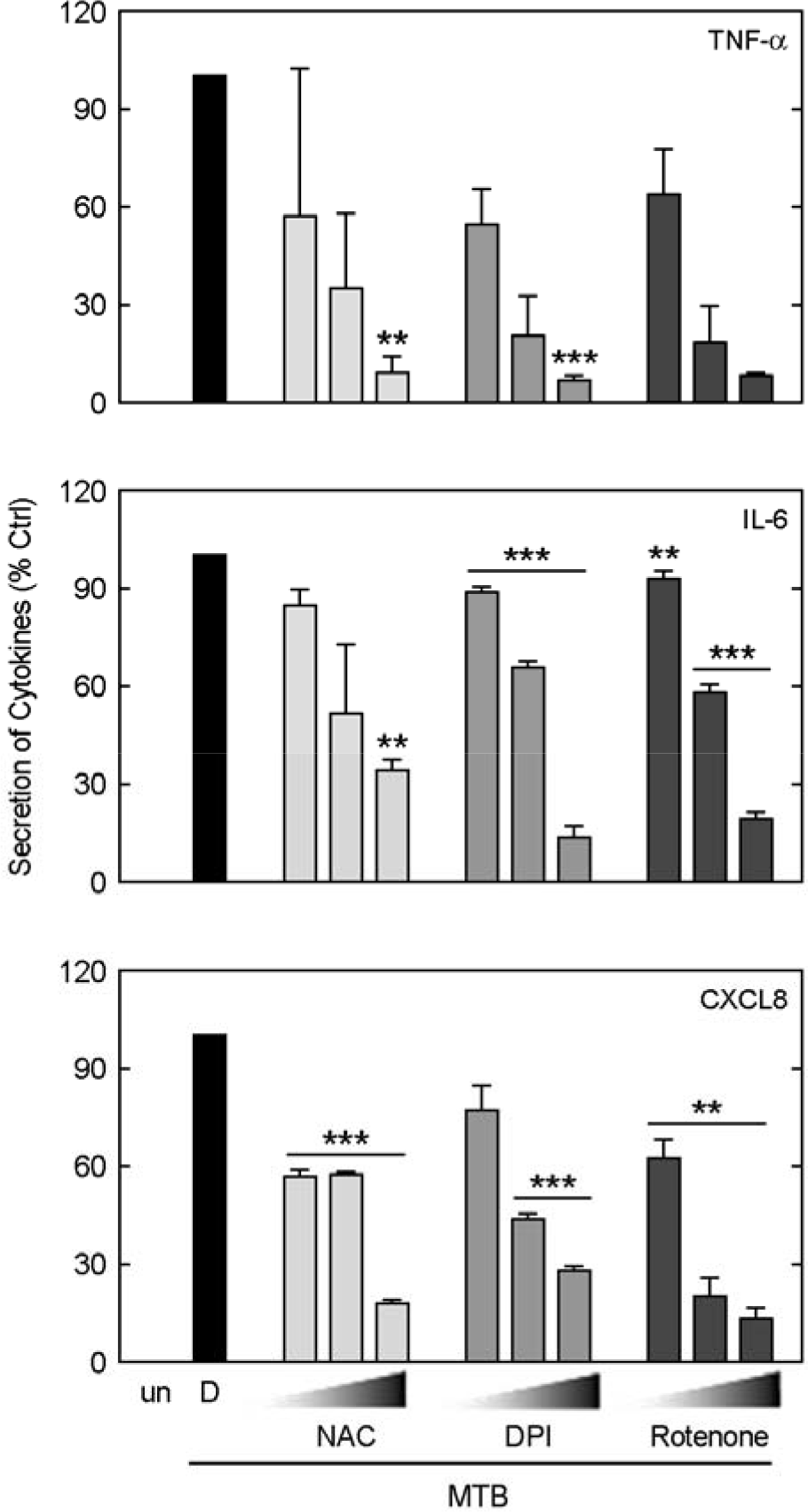 | Figure 5.The roles of ROS in pro-inflammatory cytokine and chemokine production in response to Mtb stimulation in A549 cells. The A549 cells were pretreated with NAC (10, 50, 100 mM), DPI (5, 10, 20 μM) or rotenone (5, 10, 20 μM) for 45 min, followed by stimulation with Mtb (MOI = 5) for 18 h. The supernatants were harvested at 18 h, and the production of pro-inflammatory cytokines (for TNF-α and IL-6) and chemokine (for CXCL8) was measured by ELISA. Data are the mean ± SD of three experiments. un, unstimulated; D, solvent control (0.1% DMSO). Significant differences (∗∗, p < 0.01;∗∗∗, p < 0.001) |
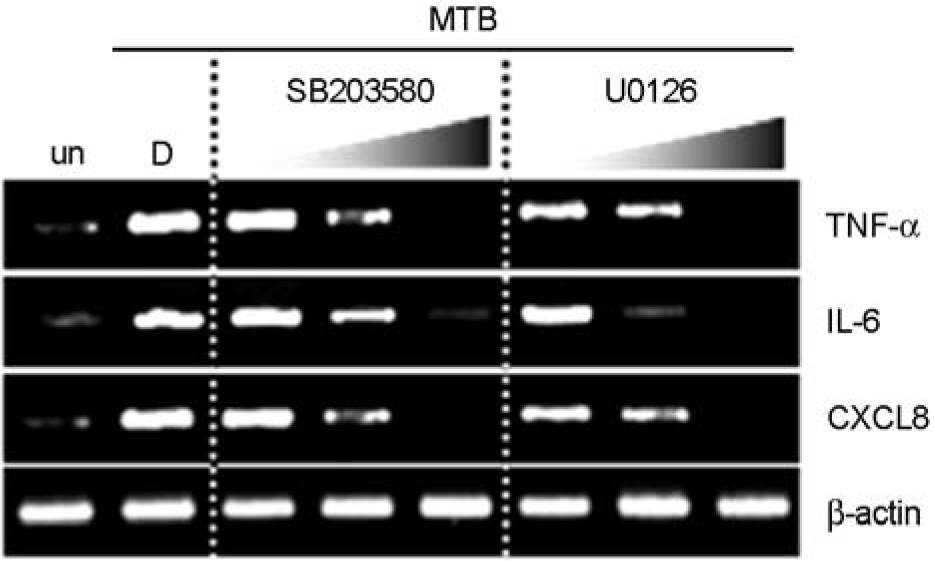 | Figure 6.The roles of MEK1/2 or p38 MAPK on the mRNA expression of TNF-α, IL-6, and CXCL8 in A549 cells. The A549 cells were pretreated in the presence or absence of U0126 (5, 10, 20 μM) or SB203580 (1, 5, 10 μM) for 45 min, and then stimulated with Mtb (MOI = 5) for 6 h. The mRNA expression of TNF-α, IL-6, and CXCL8 was detected by semi-quantitative RT-PCR analysis by using the specific primers. The data shown are representative of three experiments. un, unstimulated; D, solvent control (0.1% DMSO). |
Table 1.
Primer sequences




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download