Abstract
Prion diseases, also termed transmissible spongiform encephalopathies (TSEs), are rare and fatal neurodegenerative conditions that affect both humans and animals. Although there is increased evidence that oxidative stress plays an important role in the pathogenesis of these diseases, the direct relationship between an accumulation of abnormal prion protein (PrPSc) and the occurrence of oxidative stress has not been studied. In the present study, we have investigated the cellular localization of proteins modified by lipid peroxidation end products and its correlation with PrPSc accumulation in the brain of mice infected with the ME7 prion strain. Intense immunostaining of malondialdehyde (MDA)- and hydroxynonenal (HNE)-modified proteins were observed in the hippocampus of prion-infected mice. In serial section study, we found that these immunoreactivities were co-localized with glial fibrillary acidic protein (GFAP)-positive astrocytes as well as with PrPSc. These results clearly indicate that the heightened oxidative stress in the form of lipid peroxidation is closely associated with PrPSc accumulation in astrocytes of prion-infected mice.
Go to : 
References
1). Andreoletti O, Levavasseur E, Uro-Coste E, Tabouret G, Sarradin P, Delisle MB, Berthon P, Salvayre R, Schelcher F, Negre-Salvayre A. Astrocytes accumulate 4-hydroxynonenal adducts in murine scrapie and human Creutzfeldt-Jakob disease. Neurobiol Dis. 11:386–393. 2002.

2). Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 218:1309–1311. 1982.

3). Campbell IL, Eddleston M, Kemper P, Oldstone MB, Hobbs MV. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J Virol. 68:2383–2387. 1994.

4). Choi SI, Ju WK, Choi EK, Kim J, Lea HZ, Carp RI, Wisniewski HM, Kim YS. Mitochondrial dysfunction induced by oxidative stress in the brains of hamsters infected with the 263K scrapie agent. Acta Neuropathol. 96:279–286. 1998.
5). Choi YG, Kim JI, Jeon YC, Park SJ, Choi EK, Rubenstein R, Kascsak RJ, Carp RI, Kim YS. Nonenzymatic glycation at the N terminus of pathogenic prion protein in transmissible spongiform encephalopathies. J Biol Chem. 279:30402–30409. 2004.

6). Choi YG, Kim JI, Lee HP, Jin JK, Choi EK, Carp RI, Kim YS. Induction of heme oxygenase-1 in the brains of scrapie-infected mice. Neurosci Lett. 289:173–176. 2000.

7). Diedrich JF, Bendheim PE, Kim YS, Carp RI, Haase AT. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc Natl Acad Sci USA. 88:375–379. 1991.

8). Falsig J, Julius C, Margalith I, Schwarz P, Heppner FL, Aguzzi A. A versatile prion replication assay in organotypic brain slices. Nat Neurosci. 11:109–117. 2008.

9). Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 59:1609–1623. 1992.

10). Kascsak RJ, Tonna-DeMasi M, Fersko R, Rubenstein R, Carp RI, Powers JM. The role of antibodies to PrP in the diagnosis of transmissible spongiform encephalopathies. Dev Biol Stand. 80:141–151. 1993.
11). Kim JI, Jin JK, Choi EK, Spinner D, Rubenstein R, Carp RI, Kim YS. Increased expression and localization of cyclooxygenase-2 in astrocytes of scrapie-infected mice. J Neuroimmunol. 187:74–82. 2007.

12). Kim JI, Ju WK, Choi JH, Choi E, Carp RI, Wisniewski HM, Kim YS. Expression of cytokine genes and increased nuclear factor-kappa B activity in the brains of scrapie-infected mice. Brain Res Mol Brain Res. 73:17–27. 1999.

13). Kim NH, Park SJ, Jin JK, Kwon MS, Choi EK, Carp RI, Kim YS. Increased ferric iron content and iron-induced oxidative stress in the brains of scrapie-infected mice. Brain Res. 884:98–103. 2000.

14). Lee DW, Sohn HO, Lim HB, Lee YG, Kim YS, Carp RI, Wisniewski HM. Alteration of free radical metabolism in the brain of mice infected with scrapie agent. Free Radic Res. 30:499–507. 1999.

15). Manuelidis L, Fritch W, Xi YG. Evolution of a strain of CJD that induces BSE-like plaques. Science. 277:94–98. 1997.

16). Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, Prusiner SB. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 90:10962–10966. 1993.

17). Prusiner SB. prions. Proc Natl Acad Sci USA. 95:13363–13383. 1998.
18). Raeber AJ, Race RE, Brandner S, Priola SA, Sailer A, Bessen RA, Mucke L, Manson J, Aguzzi A, Oldstone MB, Weissmann C, Chesebro B. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 16:6057–6065. 1997.

19). Rizzardini M, Chiesa R, Angeretti N, Lucca E, Salmona M, Forloni G, Cantoni L. Prion protein fragment 106–126 differentially induces heme oxygenase-1 mRNA in cultured neurons and astroglial cells. J Neurochem. 68:715–720. 1997.

20). Toyoda K, Nagae R, Akagawa M, Ishino K, Shibata T, Ito S, Shibata N, Yamamoto T, Kobayashi M, Takasaki Y, Matsuda T, Uchida K. Protein-bound 4-hydroxy-2-nonenal: an endogenous triggering antigen of anti-DNA response. J Biol Chem. 282:25769–25778. 2007.
21). Williams A, Lucassen PJ, Ritchie D, Bruce M. PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Exp Neurol. 144:433–438. 1997.

22). Williams A, van Dam AM, Ritchie D, Eikelenboom P, Fraser H. Immunocytochemical appearance of cytokines, prostaglandin E2 and lipocortin-1 in the CNS during the incubation period of murine scrapie correlates with progressive PrP accumulations. Brain Res. 754:171–180. 1997.

23). Williams AE, van Dam AM, Man-A-Hing WK, Berkenbosch F, Eikelenboom P, Fraser H. Cytokines, prostaglandins and lipocortin-1 are present in the brains of scrapie-infected mice. Brain Res. 654:200–206. 1994.

24). Wong BS, Brown DR, Pan T, Whiteman M, Liu T, Bu X, Li R, Gambetti P, Olesik J, Rubenstein R, Sy MS. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem. 79:689–698. 2001.

Go to : 
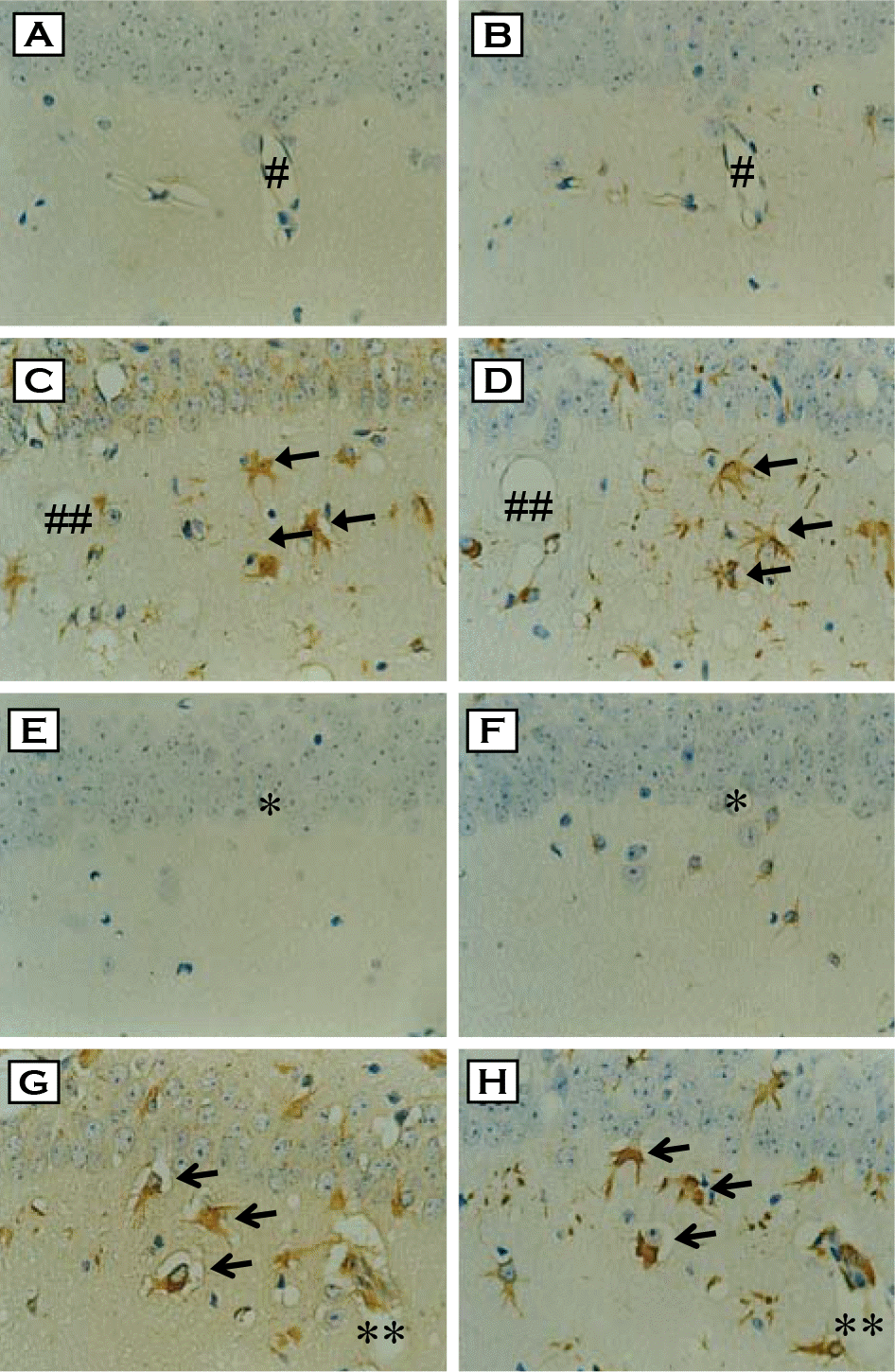 | Figure 1.Immunohistochemical staining and cellular localizations of HNE- and MDA-modified proteins in the hippocampus of ME7 prion-infected mice. Brain sections from control (A & E) and ME7-infected mice (C & G) were immunostained with either anti-HNE-modified protein (A & C) or anti-MDA-modified protein antibody (E & F). Intense immunoreactivities of HNE- and MDA-modified proteins were observed in the infected group (C & G, respectively: arrows). Each serial section was also immunostained with anti-GFAP antibody (B, D, F & H) to examine the cellular localization of HNE- and MDA-modified proteins. HNE- and MDA-modified protein-positive cells were co-localized with GFAP-positive astrocytes (C vs. D, G vs. H, respectively: arrows). Each symbol (#, ##, ∗, ∗∗) indicates landmark blood vessel in adjacent section (A & B, C & D, E & F, G & H, respectively). ×400. |
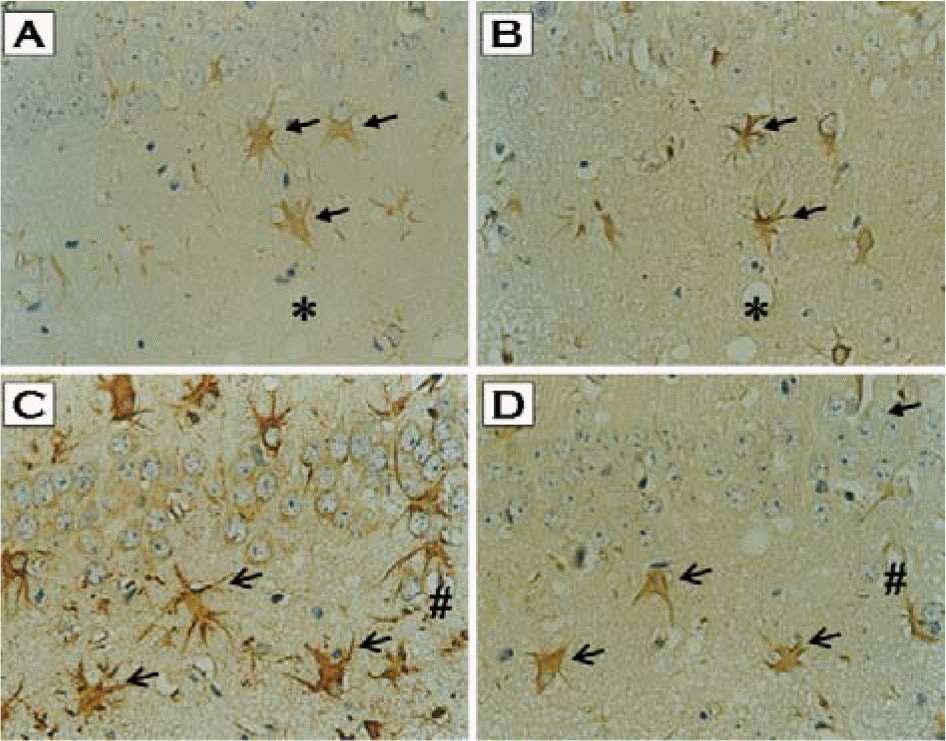 | Figure 2.Co-localization of PrPSc with either HNE- or MDA-modified proteins in the hippocampus of ME7 prion-infected mice. Brain sections from ME7-infected mice were immunostained with either anti-HNE-modified protein (A), anti-MDA-modified protein (C), or anti-PrP antibody (B & D). Immunoreactivities of HNE-modified protein as well as MDA-modified protein were colocalized with PrPSc-positive astrocytes (A vs. B, C vs. D, respectively; arrows). Each symbol (∗, #) indicates landmark blood vessel in adjacent section (A & B, C & D, respectively). ×400. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download