Abstract
It has been reported that inflammatory diseases such as pneumonitis, retinitis, and hepatitis are associated with human cytomegalovirus (HCMV). Intercellular adhesion molecule (ICAM)-1 is an important inflammatory mediator, helping monocytes adhere to endothelial cells when tissues are infected by pathogen including the HCMV. However, little is known about the mechanism of ICAM-1 stimulation by the HCMV infection in monocytes. In this study, a monocytic cell line THP-1 was used to understand ICAM-1 expression by the HCMV infection. Flow cytometric analyses demonstrated that ICAM-1 was stimulated by the HCMV in THP-1 cells with maximum at 24 hours post infection. The stimulated ICAM-1 expression was dependent on the amount of input virus. In order to understand the mechanism of ICAM-1 stimulation during the HCMV infection, cells were treated with specific inhibitors of key elements in inflammation: NF-κB inhibitor PDTC, cyclooxygenase 2 inhibitor NS398, and MEK inhibitor PD98059. Flow cytometric analyses revealed that ICAM-1 expression was decreased when treated with PDTC, but not with NS398 or PD98059. Thus, it is suggested that HCMV-induced ICAM-1 expression in THP-1 cells is associates with NF-κB.
References
1). Bolovan-Fritts CA, Mocarski ES, Wiedeman JA. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood. 93:394–398. 1999.

2). Caposio P, Dreano M, Garotta G, Gribaudo G, Landolfo S. Human cytomegalovirus stimulates cellular IKK2 activity and requires the enzyme for productive replication. J Virol. 78:3190–3195. 2004.

3). Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. Transcriptome analysis of NF-kappaB- and phosphatidylinositol 3-kinase-regulated genes in human cytomegalovirus-infected monocytes. J Virol. 82:1040–1046. 2008.
4). Chan G, Stinski MF, Guilbert LJ. Human cytomegalovirus-induced upregulation of intercellular cell adhesion molecule-1 on vilous syncytiotrophoblasts. Biol Reprod. 71:797–803. 2004.
5). Chen J, Stinski MF. Role of regulatory elements and the MAPK/ERK or p38 MAPK pathways for activation of human cytomegalovirus gene expression. J Virol. 76:4873–4885. 2002.

6). Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 62:3347–3350. 2002.
7). Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
8). Grundy JE, Downes KL. Up-regulation of LFA-3 and ICAM-1 on the surface of fibroblasts infected with Cytomegalovirus. Immunology. 78:405–412. 1993.
9). Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 95:3937–3942. 1998.

10). Ito M, Watanabe M, Ihara T, Kamiya H, Sakurai M. Increased expression of adhesion molecules (CD54, CD29 and CD44) on fibroblasts infected with cytomegalovirus. Microbiol Immunol. 39:129–133. 1995.

11). Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 12:85–98. 2000.
12). Kevil CG, Patel RP, Bullardl DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol. 281:1442–1447. 2001.

13). Knight DA, Waldman WJ, Sedmak DD. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation. 68:1814–1818. 1999.
14). Kondo K, Kaneshima H, Mocarski ES. Human cytomegalo-virus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci USA. 91:11879–11883. 1994.

15). Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/I kappa B/NF-kappa B signal cascades. Biochem Pharmacol. 69:433–449. 2005.
16). Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 77:3099–3102. 1996.

17). Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 68:4017–4021. 1994.

18). Mocarski ES Jr, Shenk T, Pass RF. Cytomegalovirus. pp.p. 2701–2772. In. Fields Virology. 5th ed.Knipe DM, Howley PM, editors. (Ed).Lippincott Williams & Wilkins;Philadelphia, USA: 2007.
19). Munro JM. Endothelial-leukocyte adhesive interactions in inflammatory diseases. Eur Heart J. 14:72–77. 1993.
20). Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L, Sweatman TW, Israel M, Arsura M. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol. 24:1823–1835. 2004.
21). Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 79:2211–2220. 2005.

22). Rodems SM, Spector DH. Extracellular Signal-Regulated Kinase Activity Is Sustained Early during Human Cytomegalo-virus Infection. J Virol. 72:9173–9180. 1998.

23). Sedmak DD, Knight DA, Vook NC, Waldman JW. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation. 58:1379–1385. 1994.
24). Smith MS, Bentz GL, Smith PM, Bivins ER, Yurochko AD. HCMV activates PI(3)K in monocytes and promotes monocyte motility and transendothelial migration in a PI(3)K-dependent manner. J Leukoc Biol. 76:65–76. 2004.

25). Smith MS, Bivins-Smith ER, Tilley AM, Bentz GL, Chan G, Minard J, Yurochko AD. Roles of phosphatidylinositol 3-kinase and NF-kappaB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence. J Virol. 81:7683–7694. 2007.
26). Söderberg-Nauclér C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 41:218–223. 2008.

27). Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 72:2059–2064. 1991.

Figure 1.
Stimulation of ICAM-1 expression by the HCMV infection on the surface of THP-1 cells. THP-1 cells were infected by HCMV TB40/E at MOI of 1 pfu/cell. Cells were harvested at indicated times and stained with PE-conjugated anti-ICAM-1 antibody. PE fluorescence was determined by a flow cytometry. (A) Flow cytometrogram of a representative experiment. C, background fluorescence; M, mock-infected cells; V, virus-infected cells. (B) Time course of ICAM-1 expression. Data are averages of three or more experiments with error bars. Open circles, mock-infected cells; closed circles, HCMV-infected cells.
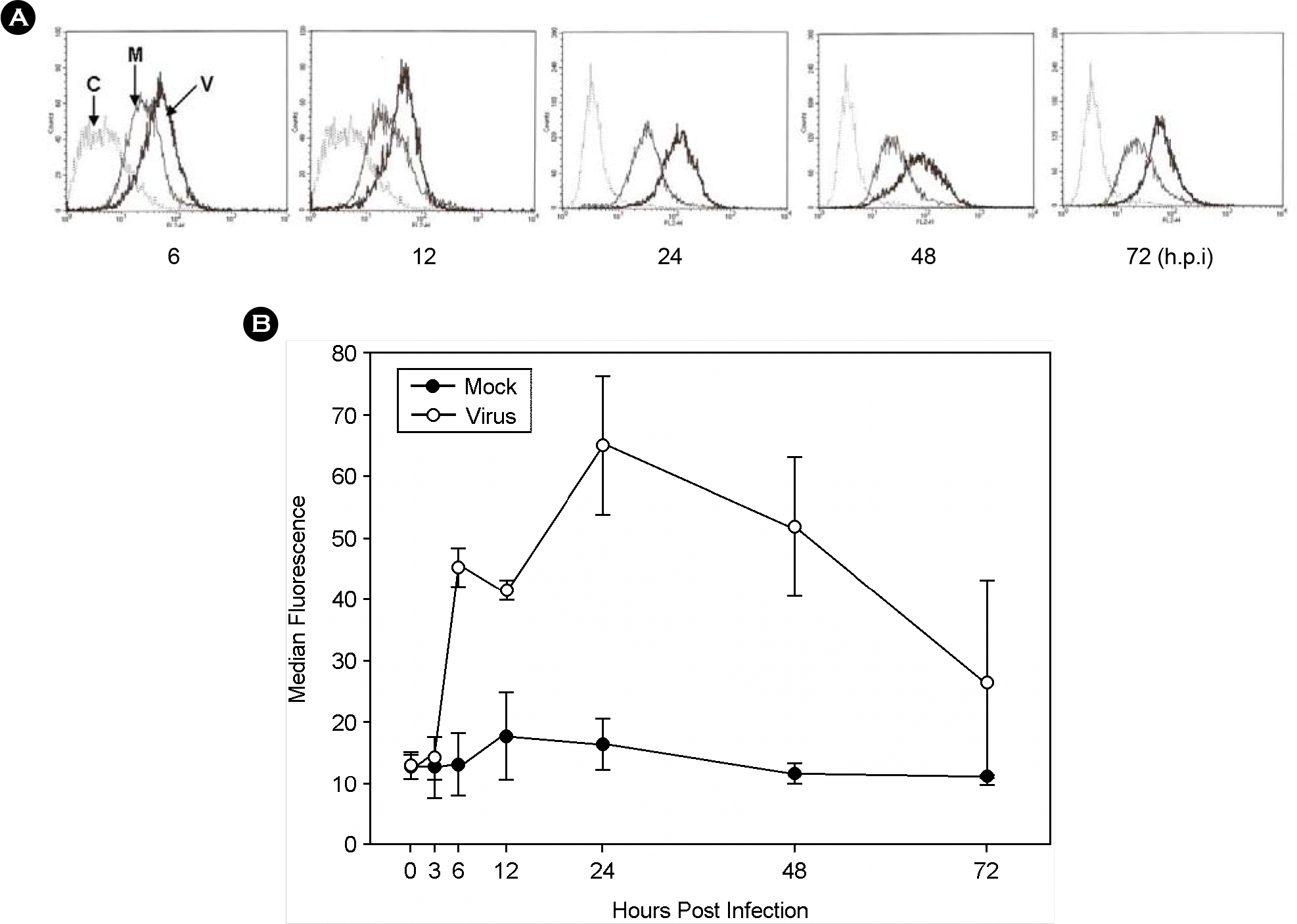
Figure 2.
HCMV MOI-dependent stimulation of ICAM-1 expression. THP-1 cells were infected with HCMV TB40/E at different MOIs. Cells were harvested at 24 hours post infection. Cells were stained with PE-conjugated anti-ICAM-1 antibody and analyzed by a flow cytometry. (A) Flow cytometrogram. C, background fluorescence; V, virus-infected cells. (B) Relative ICAM-1 expression.
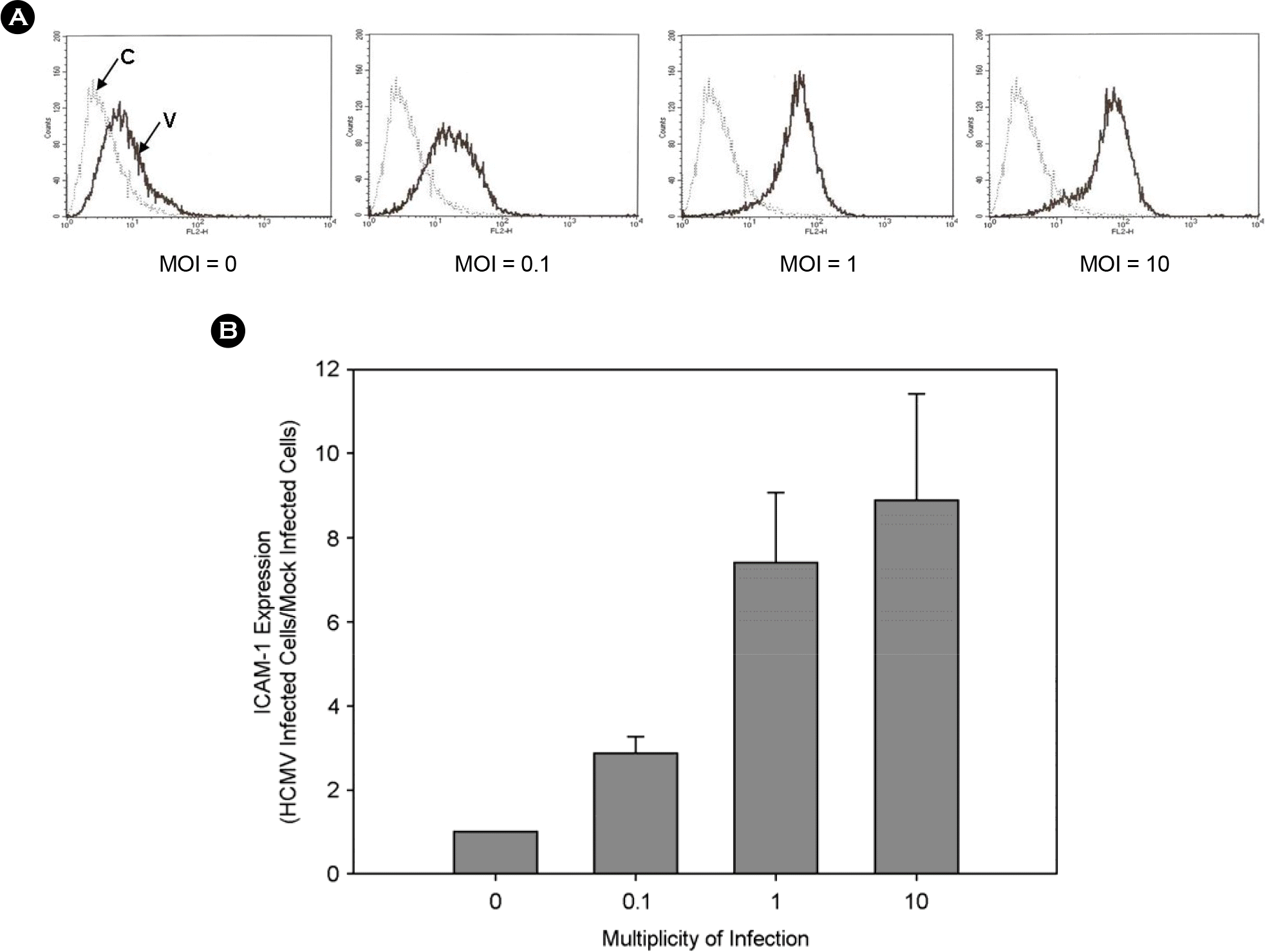
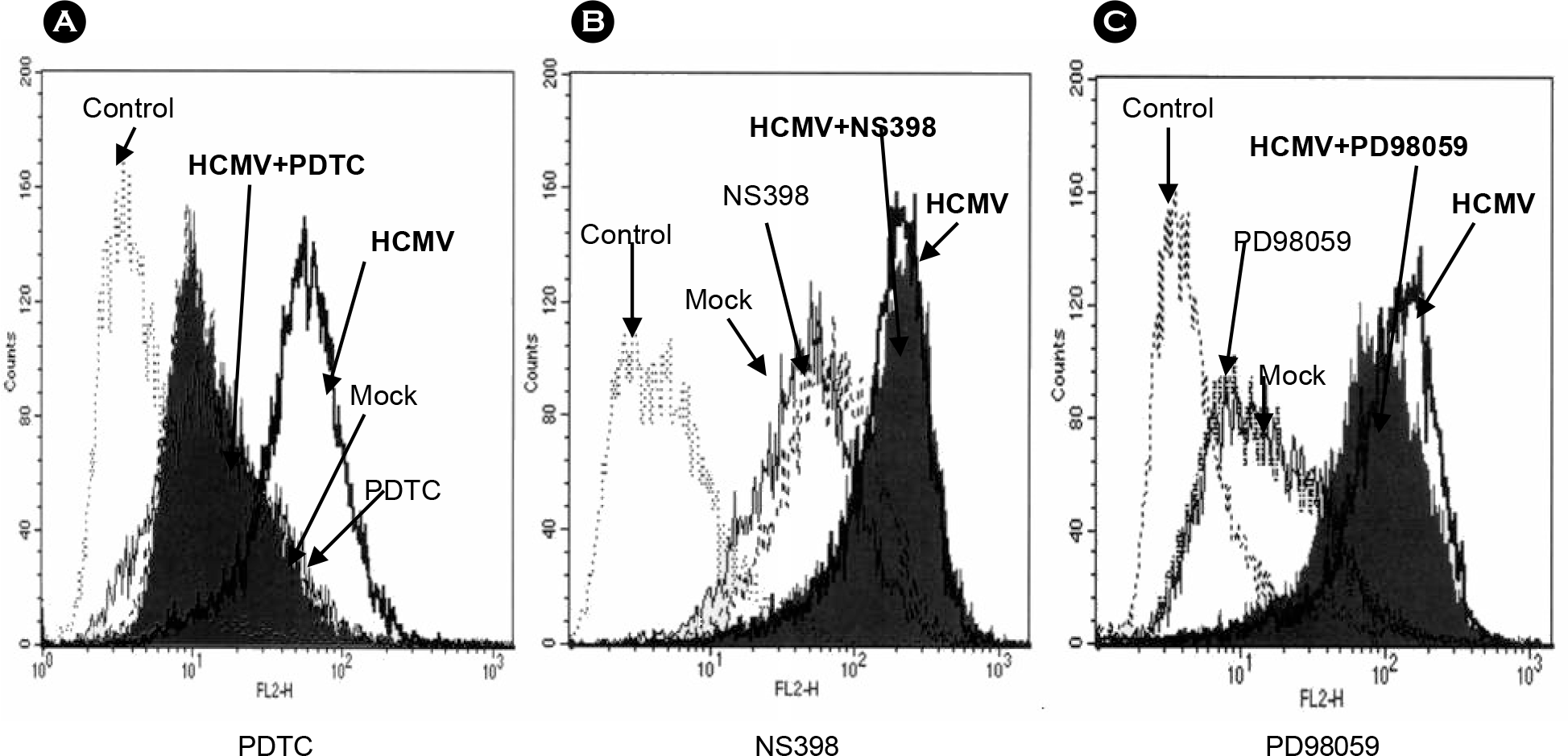
Figure 3.
Effect of specific inhibitors on the HCMV-induced ICAM-1 expression. Cells were pre-treated with inhibitors for 30 minutes and infected with HCMV TB40/E at 1 MOI. Twenty four hours after infection, cells were harvested, fixed and stained with PE-conjugated anti-ICAM-1 antibody and fluorescence was determined by a flow cytometry. (A) treated with NF-κB inhibtor PDTC (1 μM); (B) treated with COX-2 inhibitor NS398 (50 μM); (C) treated with MEK inhibtor PD98059 (50 μM).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download