Abstract
Staphylococcus aureus induces chronic infection in form of biofilm that exists in the host cells and arthroplastic prosthesis surface. In this study, the biofilm formation ability of S. aureus clinically isolated from bacteremia patients, biofilm processing and relationship of resistance to antibiotics, and difference of biofilm formation ability on different prosthetic material surfaces were studied. All of them formed biofilm and especially 6 strains of S. aureus had high ability of biofilm formation. In addition, it was found that some strains with higher biofilm formation ability make more higher polysaccharide layer production. When S. aureus ATCC 25923 forms biofilm, minimal bactericidal concentration (MBC) of biofilm bacteria is more increased than that of the planktonic state bacteria about one thousand folds. Especially, after 6 hours from starting on biofilm formation, the resistance to antibiotics was increased by more than 256 μg/ml of MBC to every antibiotics and after 8 hours prominent increase (more than 4096 μg/ml) was noted. Biofilm formation after bacterial adherence to plastic cover-slip was increased with time-dependent manner. Microcolonies were formed after 5 hours from a point that bacteria adhere to plastic cover-slip surface and after 6 hours biofilm was diffusely formed on entire surface, and then after 8 hours very thick biofilm was formed. Thicker biofilm was found on cobalt-chromium than titanium surface. These results suggest that titanium alloy materials are better than cobalt-chromium to minimize S. aureus biofilm formation on the arthroplastic material surface. Also, when microcolonies are formed after adherence of S. aureus to the arthroplastic material surface, resistance to antibiotics is starting.
Go to : 
REFERENCES
1). Akiyama H., Hamada T., Huh WK., Yamasaki O., Oono T., Fujimoto W., Iwatsuki K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br J Dermatol. 148:526–532. 2003.
2). Amorena B., Gracia E., Monzn M., Leiva J., Oteiza C., Prez M., Alabart JL., Hernndez-Yago J. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 44:43–55. 1999.
3). An YH., Friedmann RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 43:338–348. 1998.

4). Arslan S., Ozkardes F. Slime production and antibiotic susceptibility in staphylococci isolated from clinical samples. Mem Inst Oswaldo Cruz. 102:29–33. 2007.

5). Ceri H., Olson ME., Stremick C., Read RR., Morck D., Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 37:1771–1776. 1999.

6). Cramton SE., Gerke C., Schnell NF., Nichols WW., Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 67:5427–5433. 1999.
8). Fedtke I., Gtz F., Peschel A. Bacterial evasion of innate host defenses: the Staphylococcus aureus lesson. Int J Med Microbiol. 294:189–194. 2004.
9). Foster J., McDevitt D. Molecular basis of adherence of staphylococci to biomaterials. pp. p. 31–43. In. Infection associated with indwelling medical devices. 2nd ed. Bisno AL, Waldvogel FA, editors. (Ed),. American Society for Microbiology;Washington D.C.: 1994.
10). Fowler VG Jr., Fey PD., Reller LB., Chamis AL., Corey GR., Rupp ME. The intercellular adhesin locus ica is present in clinical isolates of Staphylococcus aureus from bacteremic patients with infected and uninfected prosthetic joints. Med Microbiol Immunol. 189:127–131. 2001.
11). Freeman DJ., Falkiner RF., Keane CT. New method for detecting slime production by coagulase-negative staphylococci. J Clin Pathol. 42:872–874. 1989.
12). Gallo J., Kolar M., Florschutz AV., Novotny R., Pantucek R., Kesselova M. In vitro testing of gentamicin-vancomycin loaded bone cement to prevent prosthetic joint infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 149:153–158. 2005.
14). Greenberg DP., Bayer AS., Cheung AL., Ward JI. Protective efficacy of protein A-specific antibody against bacteremic infection due to Staphylococcus aureus in an infant rat model. Infect Immun. 57:1113–1118. 1989.
15). Harris WH. Conquest of a worldwide human disease: particle-induced periprosthetic osteolysis. Clin Orthop Relat Res. 429:39–42. 2004.
16). Hussain M., Haggar A., Heilmann C., Peters G., Flock JI., Herrmann M. Insertional inactivation of Eap in Staphylococcus aureus strain Newman confers reduced staphylococcal binding to fibroblasts. Infect Immun. 70:2933–2940. 2002.
17). Jabra-Rizk MA., Meiller TF., James CE., Shirtliff ME. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 50:1463–1469. 2006.
18). Jahoda D., Sosna A., Landor I., Vavřik P., Pokorný D., Hudec T. Two-stage reimplantation using spacers-the method of choice in treatment of hip joint prosthesis-related infections. Comparison with methods used from 1979 to 1998. Acta Chir Orthop Traumatol Cech. 70:17–24. 2003.
19). Joh D., Wann ER., Kreikemeyer B., Speziale P., Höök M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211–223. 1999.

20). Krbec M., Čech O., Džupa V., Pacovský V., Klézl Z. Infection complications of total hip arthroplasty. Acta Chir Orthop Traumatol Cech. 71:179–188. 2004.
21). Lowy FD. Staphylococcus aureus infections. N Engl J Med. 339:520–532. 1998.
22). Melchior MB., Fink-Gremmels J., Gaastra W. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus plank-tonic culture. J Vet Med B Infect Dis Vet Public Health. 53:326–332. 2006.
23). Montanaro L., Arciola CR. Studying bacterial adhesion to irregular or porous surfaces. pp. p. 331–343. In. Handbook of bacterial adhesion: principles, methods and applications. An YH, Friedmann RJ, editors. (Ed),. Humana Press Inc;Totowa, NJ: 2000.

24). Neut D., van de Belt H., van Horn Jr., van der Mei HC., Busscher HJ. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 24:1829–1831. 2003.

25). Nishimura S., Tsurumoto T., Yonekura A., Adachi K., Shindo H. Antimicrobial susceptibility of Staphylococcus aureus and Staphylococcus epidermidis biofilms isolated from infected total hip arthroplasty cases. J Orthop Sci. 11:46–50. 2006.
26). Olson ME., Ceri H., Morck DW., Buret AG., Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 66:86–92. 2002.
27). Olson ME., Garvin KL., Fey PD., Rupp ME. Adherence of Staphylococcus epidermidis to biomaterials is augmented by PIA. Clin Orthop Relat Res. 451:21–24. 2006.
28). Park PW., Rosenbloom J., Abrams WR., Mecham RP. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J Biol Chem. 271:15803–15809. 1996.
29). Patti JM., Allen BL., McGavin MJ., Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 48:585–617. 1994.

30). Ramage G., Tunney MM., Patrick S., Gorman SP., Nixon JR. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 24:3221–3227. 2003.
31). Scott CP., Higham PA., Dumbleton JH. Effectiveness of bone cement containing tobramycin. J Bone Joint Surg Br. 81:440–443. 1999.

32). Shirtliff ME., Mader JT. Osteomyelitis: clinical features and molecular aspects of persistence. pp. p. 375–395. In. Persistent bacterial infections. Nataro JP, Blaser MJ, Cunningham-Rundles S, editors. (Ed),. ASM Press;Washington, D.C.: 2000.
33). Steckelberg JM., Osmon DR. Prosthetic joint infections. pp. p. 173–179. In. Infections associated with indwelling medical devices. Waldvogel FA, Bisno AL, editors. (Ed),. ASM Press;Washington, D.C.: 2000.

34). Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 292:107–113. 2002.

35). Stewart PS., Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 358:135–138. 2001.

36). Tojo M., Yamashita N., Goldmann DA., Pier GB. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 157:713–722. 1988.
37). Votava M., Woznicová V. Production of slime by staphylococcal isolates from blood cultures. Cent Eur J Public Health. 8:18–20. 2000.
38). Wahlig H., Dingeldein E. Antibiotics and bone cements. Experimental and clinical long-term observations. Acta Orthop Scand. 51:49–56. 1980.
Go to : 
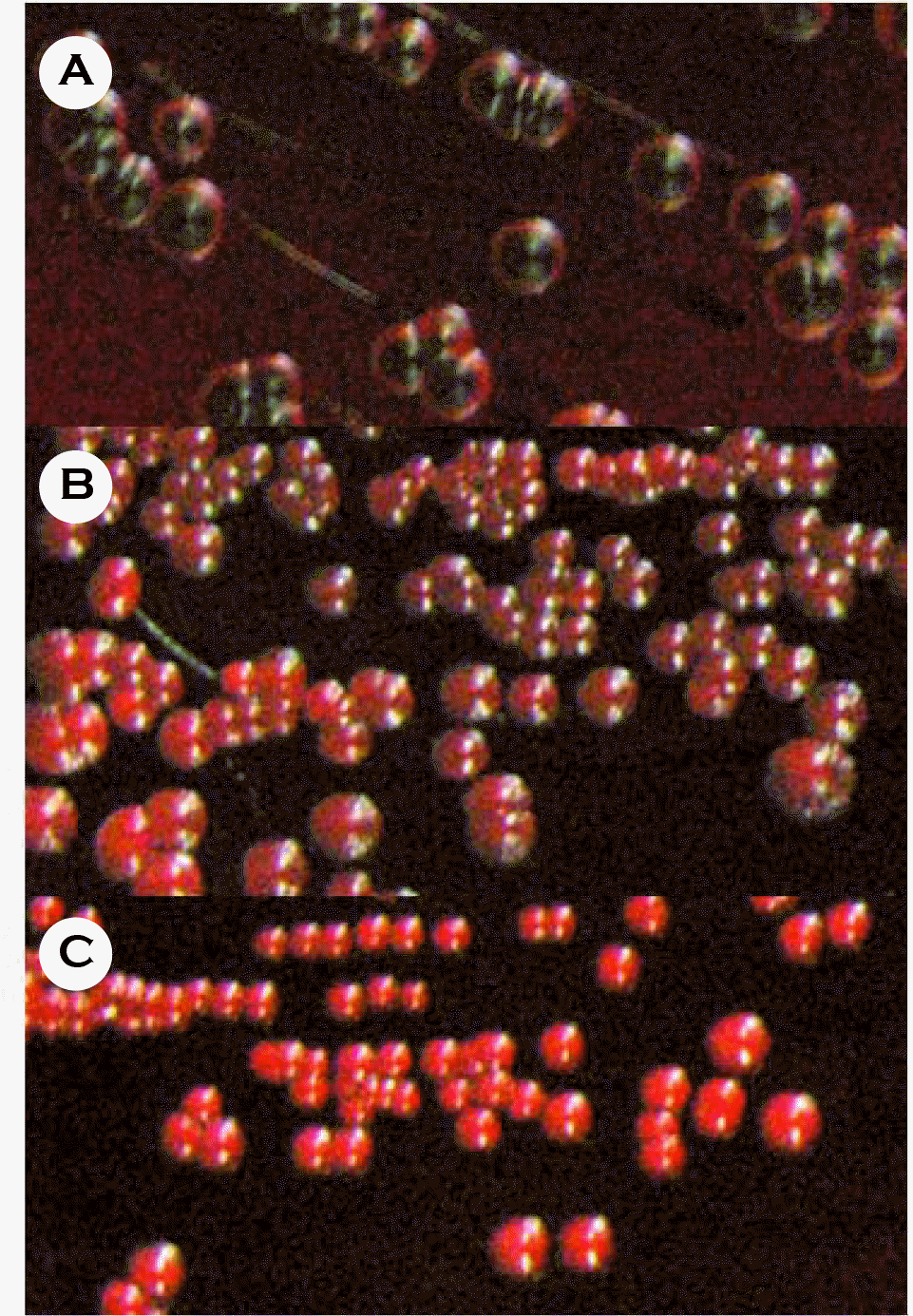 | Figure 1.Detection of glycocalyx formation ability of Staphylococcus aureus isolated from bacteremia patients. S. aureus strains were cultured on the congo red plates for 24 hrs, and colony morphology was observed. (A) Black colonies of highly-slime-producing S. aureus H05-749. (B) Bordeaux colonies of moderate-slime-producing S. aureus H05-736. (C) Red colonies of nonslime-producing S. aureus H05-735. |
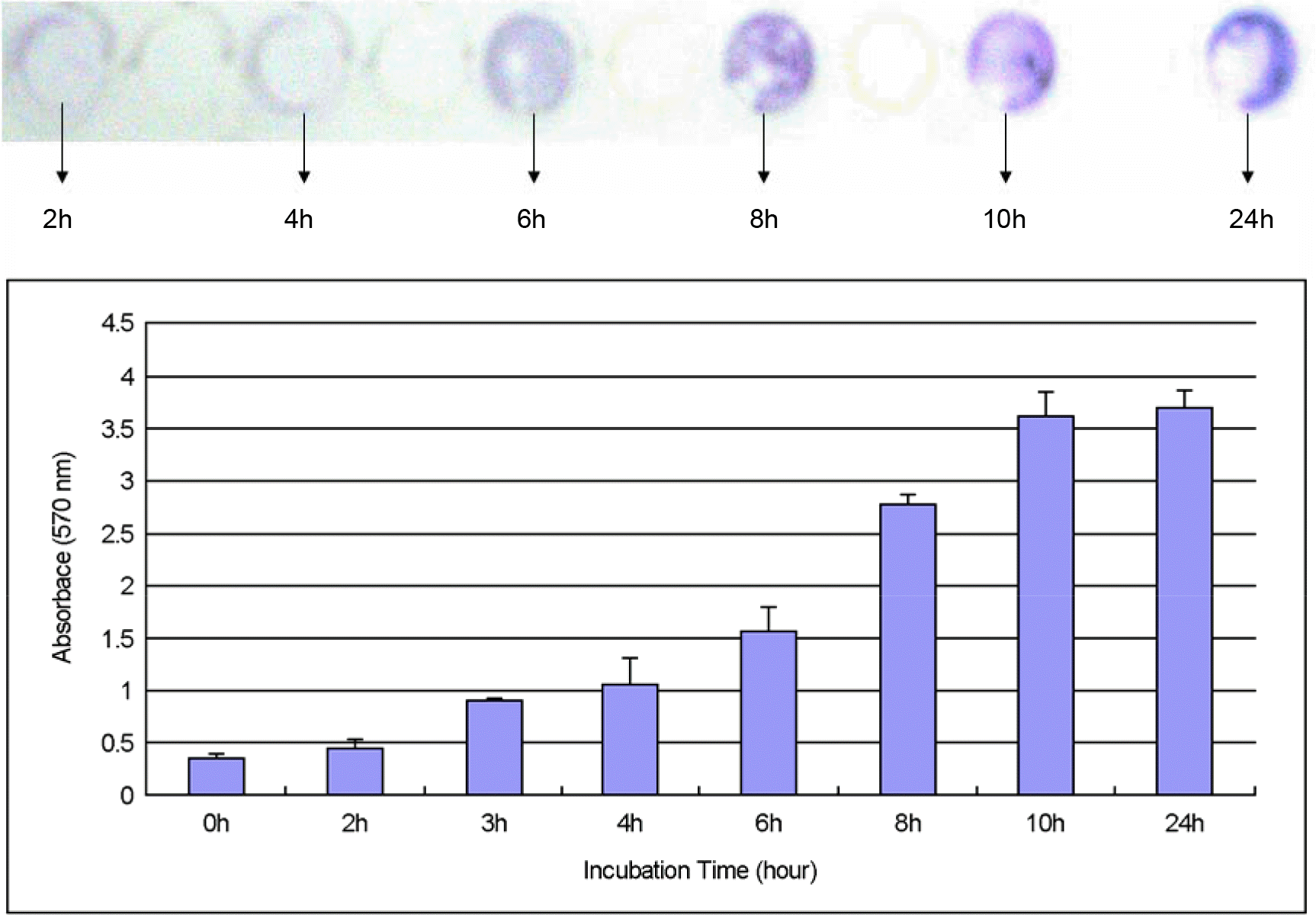 | Figure 2.Biofilm formation kinetics of S. aureus ATCC 25923 in tryptic soy broth with 0.5% glucose. A photograph of typical crystal violet-stained biofilms is shown at top of the panel and spectrophotometric results are charted with respect to time at the bottom of the panel. |
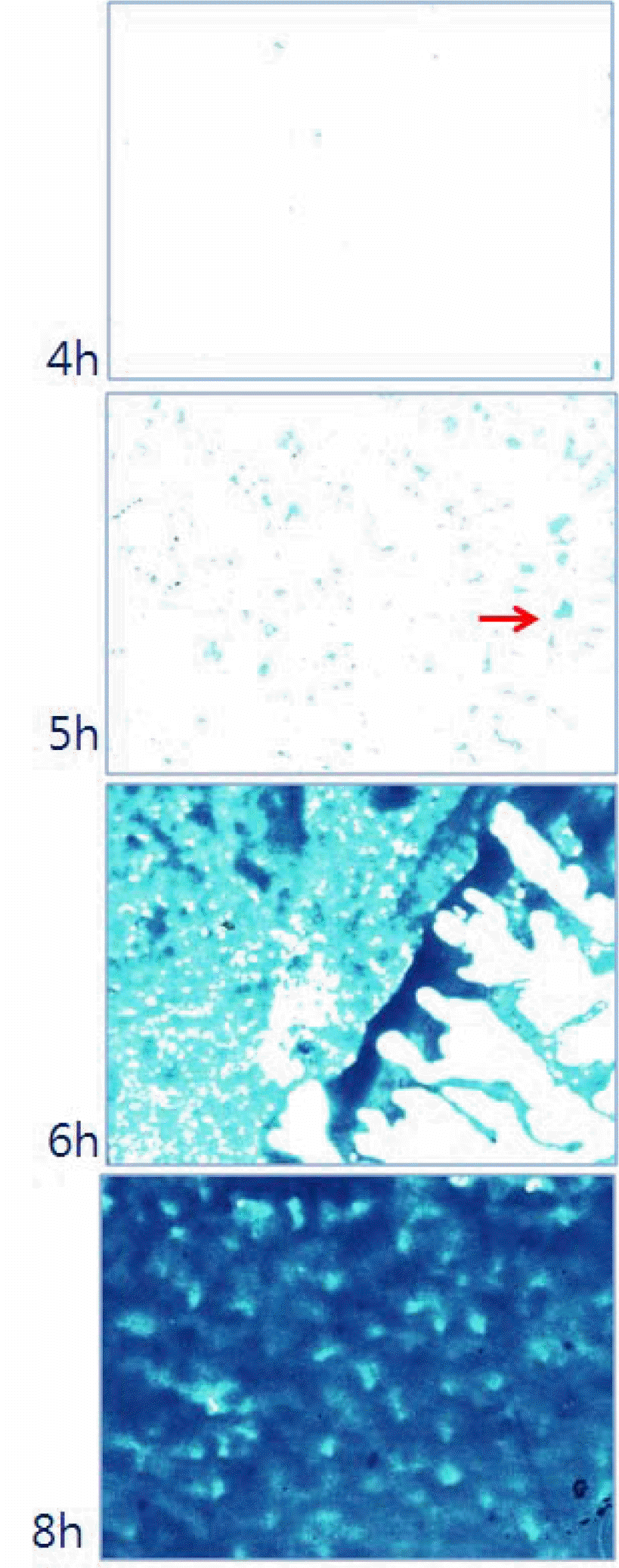 | Figure 3.Microscopic photographs of S. aureus ATCC 25923 incubated on the plastic cover-slips for 4, 5, 6, and 8 hrs (100 × magnification). Arrow indicates the microcolony of S. aureus. |
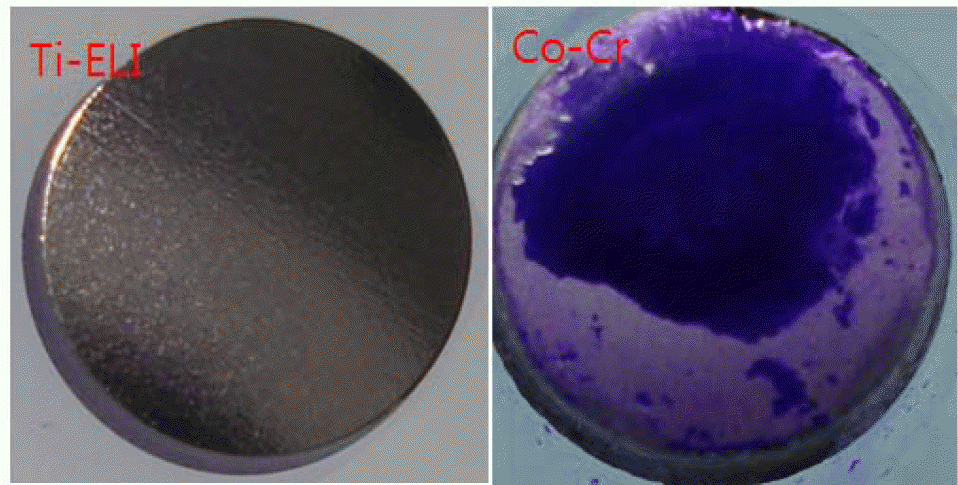 | Figure 4.Microscopic photographs of biofilm of S. aures ATCC 25923 incubated on the artificial bone surfaces for 24 hrs without shaking. Ti-ELi; titanium, Co-Cr; cobalt-chromium. |
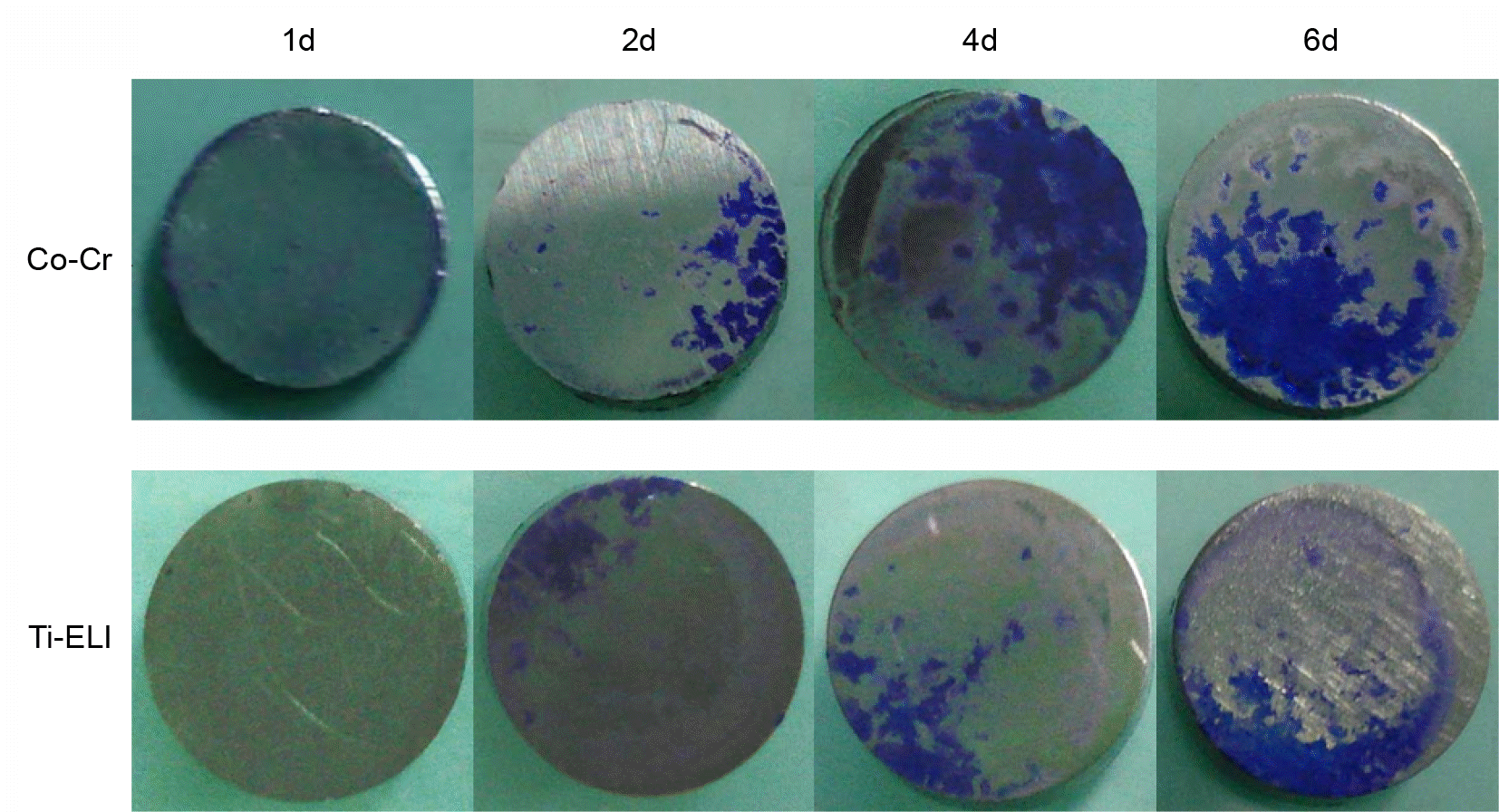 | Figure 5.Microscopic photographs of biofilm of S. aures ATCC 25923 incubated on the artificial bone surface for 1, 2, 4, and 6 days with shaking (3 × magnification). |
Table 1.
Association of biofilm production with slime production in Staphylococcus aureus isolates from bacteremia patients
| Tested strains | OD at 570 nm | Colony color on the CRA∗ |
|---|---|---|
| H05-727 | 1.276 | Bordeaux |
| H05-735 | 1.093 | Red |
| H05-736 | 1.152 | Bordeaux |
| H05-749 | 2.489 | Black |
| H05-756 | 1.007 | Bordeaux |
| H05-764 | 1.367 | Bordeaux |
| H05-774 | 2.671 | Black |
| H05-877 | 1.499 | Bordeaux |
| H05-887 | 1.361 | Bordeaux |
| H05-896 | 3.019 | Black |
| H05-914 | 1.472 | Bordeaux |
| H05-915 | 2.303 | Black |
| H05-916 | 3.919 | Black |
| H05-918 | 2.305 | Black |
| H05-929 | 1.347 | Bordeaux |
| H05-931 | 1.559 | Bordeaux |
| H05-932 | 1.105 | Bordeaux |
| ATCC14458 | 1.622 | Bordeaux |
| ATCC25923 | 2.725 | Black |
| ATCC29213 | 1.562 | Bordeaux |
Table 2.
Minimum bactericidal concentration (MBC, μg/ml) of planktonic bacteria (PB) and biofilm-forming bacteria (BB) of S. aureus ATCC 25923
| Antiicrobial agents | PB | BB |
|---|---|---|
| Clarithromycin | 1 | >2080 |
| Cefotaxime | 4 | >2080 |
| Cefmetazole | 2 | >2080 |
| Erythromycin | 4 | >2080 |
| Benzylpenicillin | 2 | >2080 |
| Vancomycin | 4 | >2080 |
Table 3.
Change of minimum bactericidal concentration (MIC, μg/ml) of biofilm-forming sessile S. aureus ATCC 25923 with the biofilm ages




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download