Abstract
Infectious bursal disease virus (IBDV) causes a highly contagious and immunosuppressive disease of chicken. Agar gel immunodiffusion using IBDV antigen extracted from bursa of Fabricius of infected chicken has been used officially for diagnosis of IBDV in Korea. In this study, in order to replace the IBDV whole virus antigen with non-infectious antigen, recombinant VP2 protein (rVP2) of IBDV was produced using recombinant baculovirus expression system. Purified baculovirus-expressed rVP2 was used as an antigen in an agar gel immunodiffusion (AGID). rVP2 antigen precipitated specifically IBDV antibodies. AGID using rVP2 antigen detected anti-IBDV antibodies from 6 dpi to 28 dpi (termination of the experiment) when specific pathogen free chickens were experimentally infected with IBDV 52/70 strain. This was consistent with result by AGID using IBDV antigen, virus neutralization test (VNT) and a commercial ELISA kit (except for one serum). The sensitivity of rVP2 was the same with that of IBDV antigen when field sera (n=324) were tested by AGID. However, AGID using rVP2 antigen detected maternal antibodies from broiler chickens (n=20) on a broiler farm up to 15 days old, although the detection rate of the AGID was relatively low compared to a commercial ELISA kit. Our results indicate that IBDV whole virus antigen from IBDV infected chickens would be replaced with recombinant VP2 protein as an antigen for AGID.
REFERENCES
1). 이영옥, 김순재, 최정옥, 전무상, 박근식: 국내종계의 infectious bursal disease virus 의 감염상황 및 분리주의 생물학적 특성. 농사시험보고서 (축산, 가축위생편). 23:136–142. 1981.
2). Böttcher B., Kiselev NA., Stel'Mashchuk VY., Perevozchikova NA., Borisov AV., Crowther RA. Three-dimensional structure of infectious bursal disease virus determined by electron cryomicroscopy. J Virol. 71:325–330. 1997.

3). Brandt M., Yao K., Liu M., Heckert RA., Vakharia VN. Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. J Virol. 75:11974–11982. 2001.

4). Cullen GA., Wyeth PJ. Quantitation of antibodies to infectious bursal disease. Vet Rec. 97:315. 1975.

5). Delmas B., Kibenge FSB., Leong JC., Mundt E., Vakharia VN., Wu JL. Birnaviridae. pp. p. 561–569. In. Virus Taxonomy. Eighth report of the International Committee on Taxonomy of Viruses. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. (Ed),. Academic Press;London: 2004.
6). Fahey KJ., Erny K., Crooks J. A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J Gen Virol. 70:1473–1481. 1989.

7). Fahey KJ., McWaters P., Brown MA., Erny K., Murphy VJ., Hewish DR. Virus-neutralizing and passively protective monoclonal antibodies to infectious bursal disease virus of chickens. Avian Dis. 35:365–373. 1991.

8). Francois A., Chevalier C., Delmas B., Eterradossi N., Toquin D., Rivallan G., Langlois P. Avian adenovirus CELO recombinants expressing VP2 of infectious bursal disease virus induce protection against bursal disease in chickens. Vaccine. 22:2351–2360. 2004.

9). Gagic M., Lazic S., Asanin R., Kapetanov M. Production of the antigen of infectious bursal disease virus (IBDV) for the agar-gel precipitin test and its sensitivity compared to the virus neutralization test and ELISA. Acta Veterinaria. 46:95–102. 1996.
10). Heine HG., Boyle DB. Infectious bursal disease virus structural protein VP2 expressed by a fowlpox virus recombinant confers protection against disease in chickens. Arch Virol. 131:277–292. 1993.
11). Ismail NM., Saif YM., Moorhead PD. Lack of pathogenicity of five serotype 2 infectious bursal disease viruses in chickens. Avian Dis. 32:757–759. 1988.

12). Jackwood DJ., Henderson KS., Jackwood RJ. Enzyme-linked immunosorbent assay-based detection of antibodies to antigenic subtypes of infectious bursal disease viruses of chickens. Clin Diagn Lab Immunol. 3:456–463. 1996.

13). Jackwood DJ., Saif YM., Moorhead PD. Immunogenicity and antigenicity of infectious disease virus serotypes I and II in chickens. Avian Dis. 29:1184–1194. 1985.
14). Kim TK., Yeo SG. Cloning and nucleotide analysis of segment A gene of infectious bursal disease virus detected in Korea. Virus Genes. 26:97–106. 2003.
15). Kwon HM., Kim DK., Hahn TW., Han JH., Jackwood DJ. Sequence of precursor polyprotein gene (segment A) of infectious bursal disease viruses isolated in Korea. Avian Dis. 44:691–696. 2000.

16). Kwon HM., Kim DK., Seong HW. Sequence analysis of the variable VP2 gene of infectious bursal disease viruses isolated in Korea. Korean J Vet Res. 39:545–553. 1999.
17). Kwon YK., Mo IP., Seong HW., Kang MI., Koh HB., Lee JG., Yang CK. Studies on the pathogenicity of infectious bursal disease virus (SH/92) isolated in Korea. RDA J Agri Sci. 37:637–647. 1995.
18). Lukert PD., Saif YM. Infectious bursal disease. pp. p. 721–738. In. Disease of poultry. 10th ed. Calnek BD, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. (Ed),. Mosby-Wolfe;Ames, Iowa: 1997.
19). Marquardt WW., Johnson RB., Odenwald WF., Schlotthober BA. An indirect enzyme-linked immunoassay (ELISA) for measuring antibodies in chickens infected with infectious bursal disease virus. Avian Dis. 24:375–385. 1980.
20). McFerran JB., McNulty MS., McKillop ER., Conner TJ., McCracken RM., Collins DS., Allan GM. Isolation and serological studies with infectious bursal diseases from fowl, turkey and duck: demonstration of a second serotype. Avian Pathol. 9:395–404. 1980.
21). Omar AR., Kim CL., Bejo MH., Ideris A. Efficacy of VP2 protein expressed in E. coli for protection against highly virulent infectious bursal disease virus. J Vet Sci. 7:241–247. 2006.
22). Pitcovski J., Di-Castro D., Shaaltiel Y., Azriel A., Gutter B., Yarkoni E., Michael A., Krispel S., Levi BZ. Insect cell-derived VP2 of infectious bursal disease virus confers protection against the disease in chickens. Avian Dis. 40:753–761. 1996.

23). Pitcovski J., Gutter B., Gallili G., Goldway M., Perelman B., Gross G., Krispel S., Barbakov M., Michael A. Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine. 21:4736–4743. 2003.

24). Rodriguez-Chavez IR., Rosenberger JK., Cloud SS., Pope CR. Characterization of the antigenic, immunogenic, and pathogenic variation of infectious bursal disease virus due to propagation in different host systems (bursa, embryo, and cell culture), III: pathogenicity. Avian Pathol. 31:485–492. 2002.

25). Rong J., Cheng T., Liu X., Jiang T., Gu H., Zou G. Development of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine. 23:4844–4851. 2005.

26). Sharma JM., Dohms JE., Metz AL. Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of IBDV and their effect on humoral and cellular immune competence of specific-pathogen-free chickens. Avian Dis. 33:112–124. 1989.
27). van den Berg TP. Acute infectious bursal disease in poultry: a review. Avian Pathol. 29:175–194. 2000.
28). van den Berg TP., Morales D., Eterradossi N., Rivallan G., Toquin D., Raue R., Zierenberg K., Zhang MF., Zhu YP., Wang CQ., Zheng HJ., Wang X., Chen GC., Lim BL., Muller H. Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathol. 33:470–476. 2004.

Figure 1.
Immunofluorescence staining of IBDV VP2 protein expression. The Sf9 cells infected with recombinant baculovirus (Bac/IBDVP2) were stained using chicken anti-IBDV antiserum (A), IBDV-specific monoclonal antibody R63 (B) and IBDV antibody negative chicken serum (C).
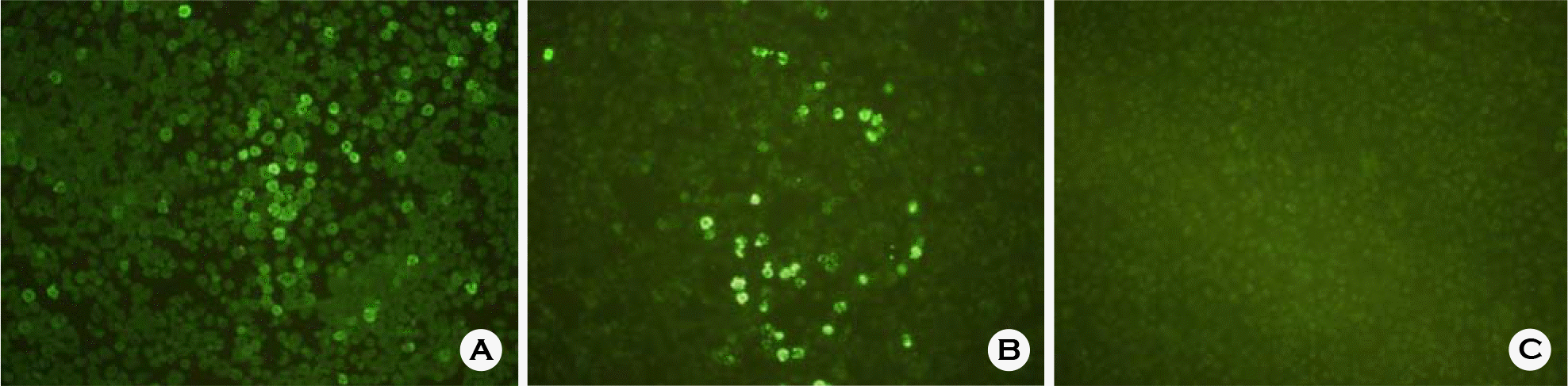
Figure 2.
Analysis of purified IBDV VP2 protein expressed from Sf9 cells by recombinant baculovirus (Bac/IBDVP2). A: SDS-PAGE analysis of purified IBDV VP2 protein fractions F1 (103 μg/ml), F2 (665 μg/ml), F3 (275 μg/ml) and F4 (98 μg/ml); B: Western blot analysis of purified IBDV VP2 protein fraction F2 using IBDV VP2-specific monoclonal antibody R63 (lane 1) and VP3-specific monoclonal antibody B29 (lane 2).
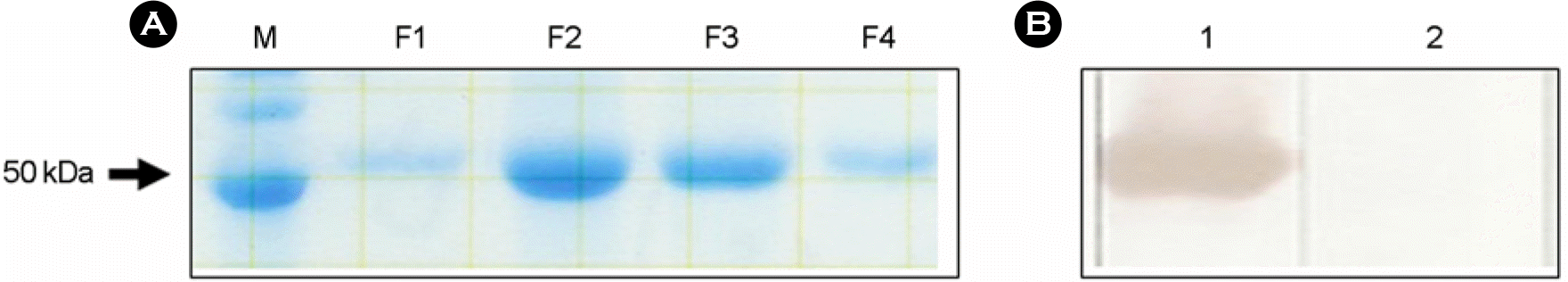
Figure 3.
Titration of IBDV (A) or recombinant IBDV VP2 protein (B) by sandwich ELISA using monoclonal antibody R63 and anti-IBDV chicken serum.
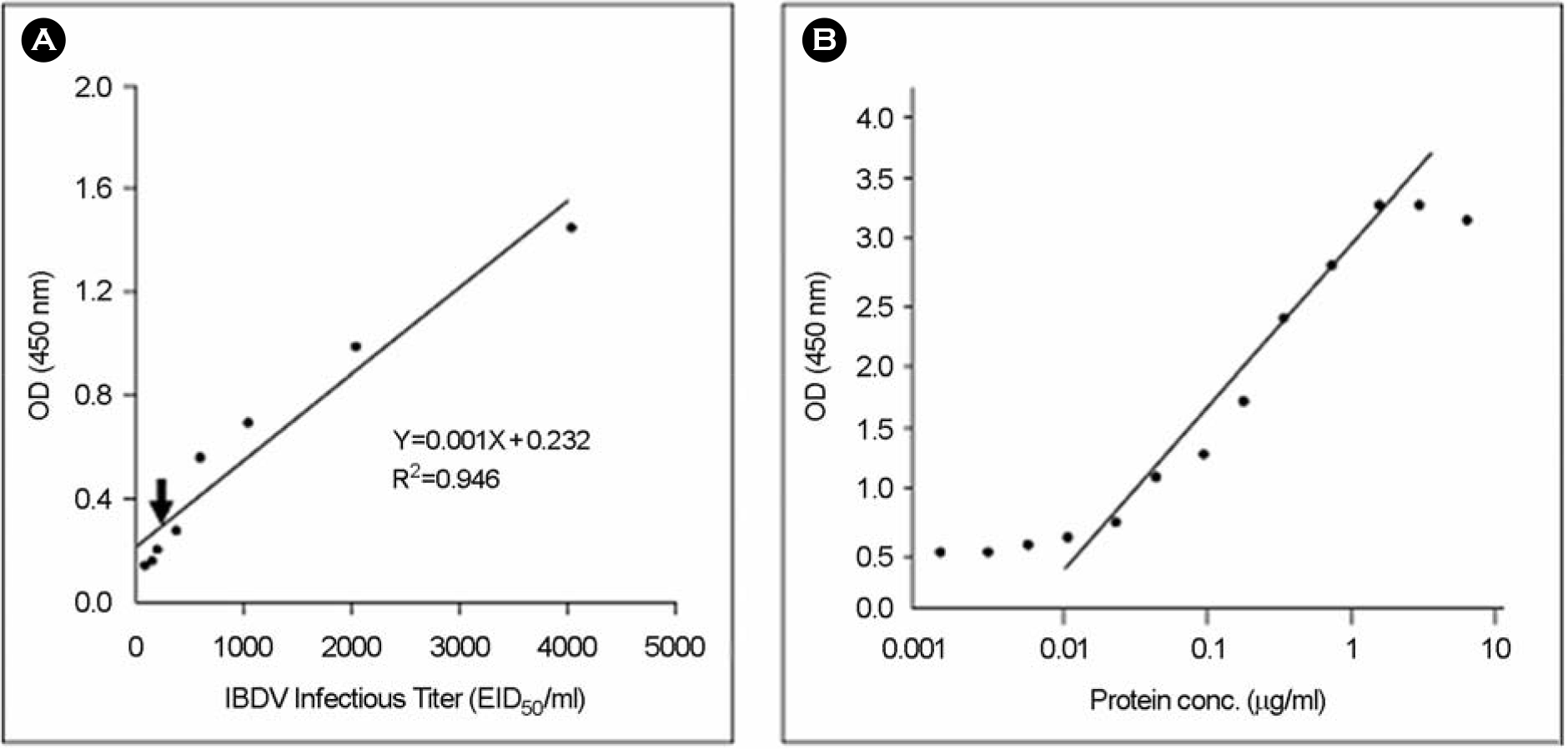
Figure 4.
Detection of anti-IBDV maternal antibodies in broiler measured by AGID test using recombinant IBDV VP2 protein.
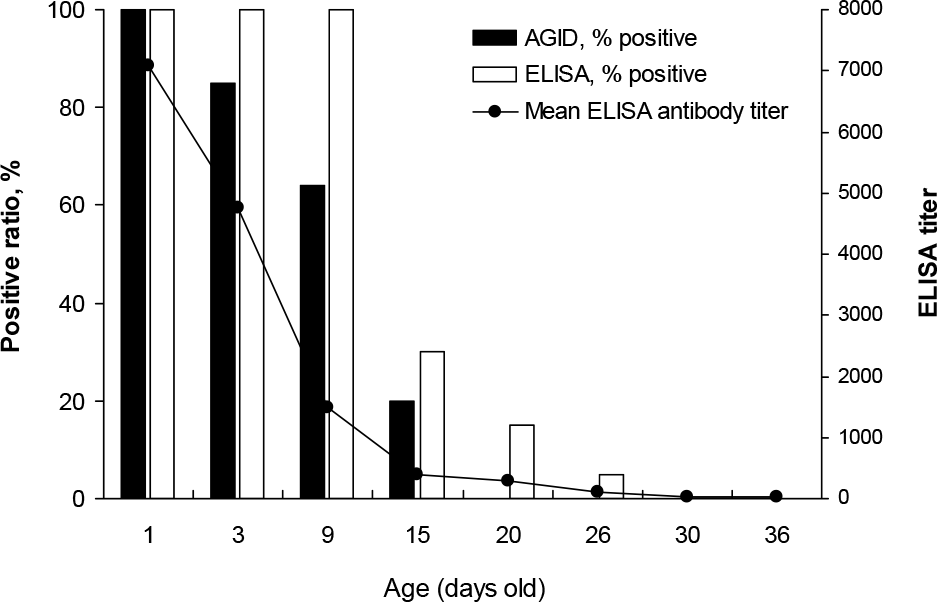
Table 1.
Detection of anti-IBDV antibodies in SPF chickens following experimental infection by AGID assays
| DPI | ELISAa | VNTb | AGID | |
|---|---|---|---|---|
| IBDV | rVP2 | |||
| 0 | 0/10c | 0/10 | 0/10 | 0/10 |
| 3 | 0/4 | 0/4 | 0/4 | 0/4 |
| 6 | 6/6 | 5/6 | 5/6 | 5/6 |
| 9 | 6/6 | 6/6 | 6/6 | 6/6 |
| 14 | 7/7 | 7/7 | 7/7 | 7/7 |
| 28 | 7/7 | 7/7 | 7/7 | 7/7 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download