Abstract
Mycobacterium abscessus has been identified as an emerging pulmonary pathogen in humans. Previously, it was documented that a spontaneously formed rough variant of M. abscessus causes persistent and invasive infection in mice, while a smooth isogenic variant does not. However, little is known for immune responses elicited by M. abscessus variants artificially induced by culture conditions and their culture filtrate antigens. Thus, morphological variants of M. abscessus type strain (ATCC19977T) were generated by an acidic and low oxygen culture conditions. Overall comparison between the variant and its original smooth strain showed that the rough variant was less virulent than original smooth strain in murine bone-marrow derived macrophage. To understand the basis for the difference, the protein expression pattern in the culture filtrates of each strain was analyzed by 1-dimensional electrophoresis. Generally, the protein expressions were more influenced by pH conditions than oxygen pressures. Interestingly, several proteins, mainly lower than 30 kDa molecular weight, were uniquely expressed in normal culture conditions. In contrast, several high molecular weight proteins (>55 kDa) were induced by acidic and low oxygen culture conditions. These findings not only provide new insights of association between morphological change and the virulence, but may also be useful in the design of immunological diagnosis and vaccines for M. abscessus infection.
Go to : 
REFERENCES
1). Bartralot R., Pujol RM., Garcia-Patos V., Sitjas D., Martin-Casabona N., Coll P., Alomar A., Castells A. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. Comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 27:124–129. 2000.

2). Belisle JT., Brennan PJ. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 171:3465–3470. 1989.

3). Bermudez LE., Petrofsky M. Regulation of the expression of Mycobacterium avium complex proteins differs according to the environment within host cells. Immunol Cell Biol. 75:35–40. 1997.
4). Blossom DB., Alelis KA., Chang DC., Flores AH., Gill J., Beall D., Peterson AM., Jensen B., Noble-Wang J., Williams M., Yakrus MA., Arduino MJ., Srinivasan A. Pseudooutbreak of Mycobacterium abscessus Infection Caused by laboratory contamination. Infect Control Hosp Epidemiol. 29:57–62. 2008.
5). Byrd TF. Tumor necrosis factor alpha (TNFalpha) promotes growth of virulent Mycobacterium tuberculosis in human monocytes iron-mediated growth suppression is correlated with decreased release of TNFalpha from iron-treated infected monocytes. J Clin Invest. 99:2518–2529. 1997.
6). Byrd TF., Lyons CR. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun. 67:4700–4707. 1999.
7). Catherinot E., Clarissou J., Etienne G., Ripoll F., Emile JF., Daffé M., Perronne C., Soudais C., Gaillard JL., Rottman M. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect Immun. 75:1055–1058. 2007.
8). Eckstein TM., Inamine JM., Lambert ML., Belisle JT. A genetic mechanism for deletion of the ser2 gene cluster and formation of rough morphological variants of Mycobacterium avium. J Bacteriol. 182:6177–6182. 2000.
9). Etienne G., Villeneuve C., Billman-Jacobe H., Astarie-Dequeker C., Dupont MA., Daffé M. The impact of the absence of glycopeptidolipids on the ultrastructure, cell surface and cell wall properties, and phagocytosis of Mycobacterium smegmatis. Microbiology. 148:3089–3100. 2002.
10). Hayes D Jr. Mycobacterium abscessus and other non-tuberculous mycobacteria: evolving respiratory pathogens in cystic fibrosis: a case report and review. South Med J. 98:657–661. 2005.
11). Howard ST., Rhoades E., Recht J., Pang X., Alsup A., Kolter R., Lyons CR., Byrd TF. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 152:1581–1590. 2006.
12). Hunter RL., Venkataprasad N., Olsen MR. The role of trehalose dimycolate (cord factor) on morphology of virulent M. tuberculosis in vitro. Tuberculosis (Edinb). 86:349–356. 2006.
13). Irani VR., Maslow JN. Induction of murine macrophage TNF-alpha synthesis by Mycobacterium avium is modulated through complement-dependent interaction via complement receptors 3 and 4 in relation to M. avium glycopeptidolipid. FEMS Microbiol Lett. 246:221–228. 2005.
14). Jeon K., Koh WJ., Kwon OJ., Suh GY., Chung MP., Kim H., Lee NY., Park YK., Bai GH. Recovery rate of NTM from AFB smear-positive sputum specimens at a medical centre in South Korea. Int J Tuberc Lung Dis. 9:1046–1051. 2005.
15). Koh WJ., Kwon OJ., Jeon K., Kim TS., Lee KS., Park YK., Bai GH. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 129:341–348. 2006.

16). Koh WJ., Kwon OJ., Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 3:145–157. 2002.

17). Kusunoki S., Ezaki T. Proposal of Mycobacterium peregrinum sp. nov., nom. rev., and elevation of Mycobacterium chelonae subsp. abscessus (Kubica et al.) to species status: Mycobacterium abscessus comb. nov. Int J Syst Bacteriol. 42:240–245. 1992.
18). Le Bourgeois M., Sermet-Gaudelus I., Catherinot E., Gaillard JL. Nontuberculous mycobacteria in cystic fibrosis. Arch Pediatr. 12:S117–S121. 2005.
19). Manca C., Tsenova L., Freeman S., Barczak AK., Tovey M., Murray PJ., Barry C., Kaplan G. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 25:694–701. 2005.
20). Marras TK., Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 23:553–567. 2002.
21). Mustafa AS. Development of new vaccines and diagnostic reagents against tuberculosis. Mol Immunol. 39:113–119. 2002.

22). Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS. 114:319–328. 2006.
23). Ripoll F., Deshayes C., Pasek S., Laval F., Beretti JL., Biet F., Risler JL., Daffé M., Etienne G., Gaillard JL., Reyrat JM. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics. 8:114. 2007.

24). Sampaio EP., Elloumi HZ., Zelazny A., Ding L., Paulson ML., Sher A., Bafica AL., Shea YR., Holland SM. M. abscessus and M. avium Trigger Toll like Receptor 2 and Distinct Cytokine Response in Human Cells. Am J Respir Cell Mol Biol [Epub ahead of print]. 2008.
25). Sharma K., Chopra P., Singh Y. Recent advances towards identification of new drug targets for Mycobacterium tuberculosis. Expert Opin Ther Targets. 8:79–93. 2004.
26). Shin DM., Yang CS., Yuk JM., Lee JY., Kim KH., Shin SJ., Takahara K., Lee SJ., Jo EK. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol [Epub ahead of print]. 2008.
27). Torrelles JB., Ellis D., Osborne T., Hoefer A., Orme IM., Chatterjee D., Brennan PJ., Cooper AM. Characterization of virulence, colony morphotype and the glycopeptidolipid of Mycobacterium avium strain 104. Tuberculosis (Edinb). 82:293–300. 2002.
28). Villeneuve C., Etienne G., Abadie V., Montrozier H., Bordier C., Laval F., Daffe M., Maridonneau-Parini I., Astarie-Dequeker C. Surface-exposed glycopeptidolipids of Mycobacterium smegmatis specifically inhibit the phagocytosis of mycobacteria by human macrophages. Identification of a novel family of glycopeptidolipids. J Biol Chem. 278:51291–51300. 2003.
Go to : 
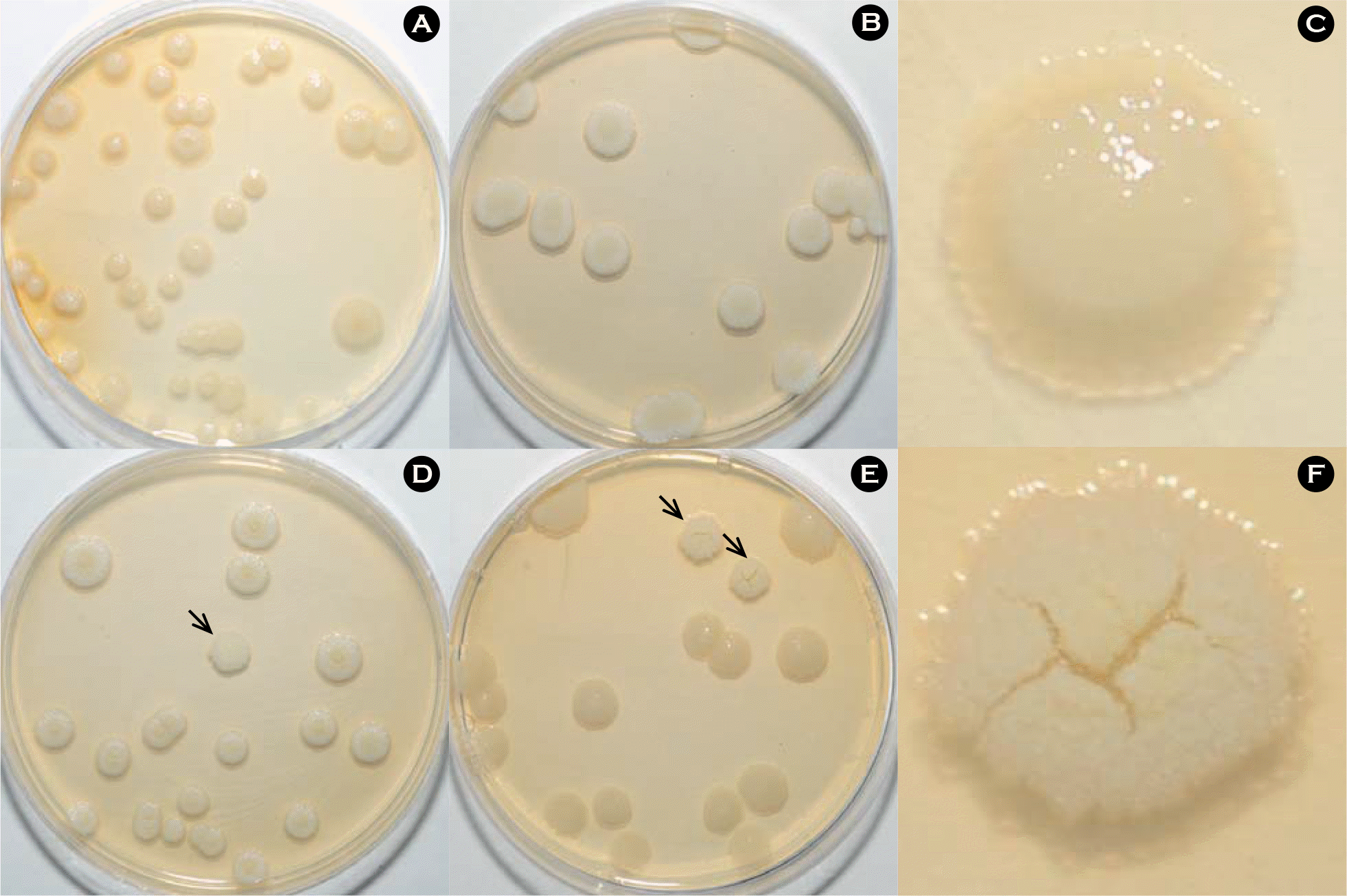 | Figure 1.Colony morphology of M. abscessus type strain under variable culture conditions. (A) Normal oxygen tension (21%) and pH 6.0, (B) pH 7.5, (D) pH 5.0 and (E) low oxygen tension (13%), pH 5.0 culture conditions affected the morphology of M. abscessus. The smooth (C) and rough (F) variants were obtained from modified culture conditions after 7 days of incubation on 7H10 agar plate at 37°C. The arrows indicate rough variants of M. abscessus. |
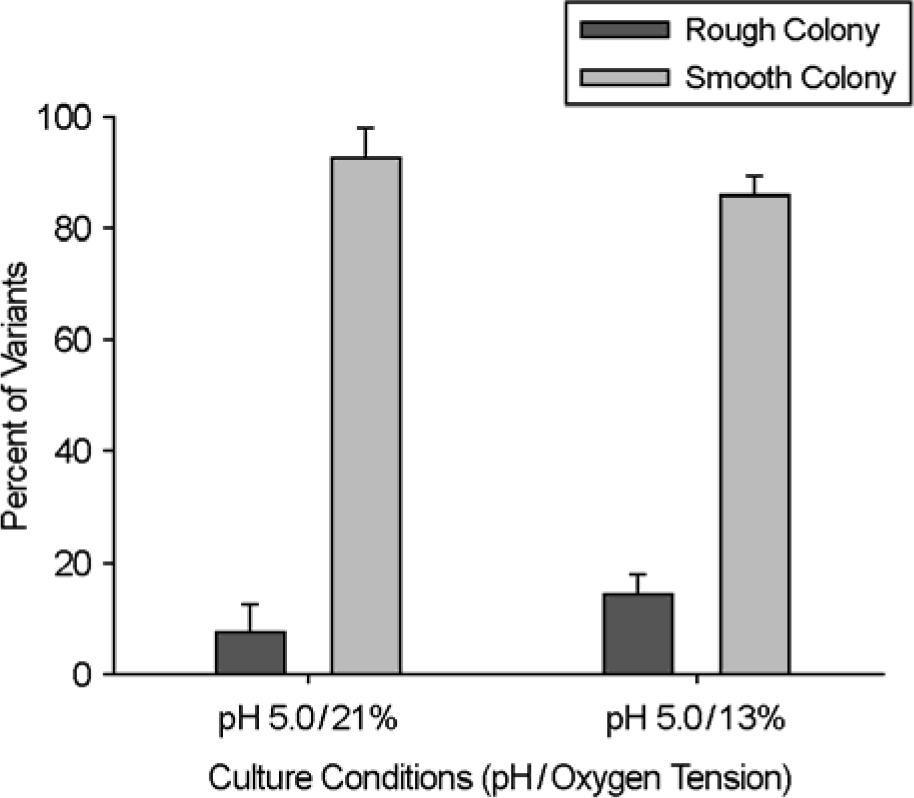 | Figure 2.The frequency of morphotype changes under acidic and low oxygen tension culture conditions. In vitro conditional culture system affects the colony morphology of M. abscessus. Rough colonies of M. abscessus have mutated from smooth type strain. |
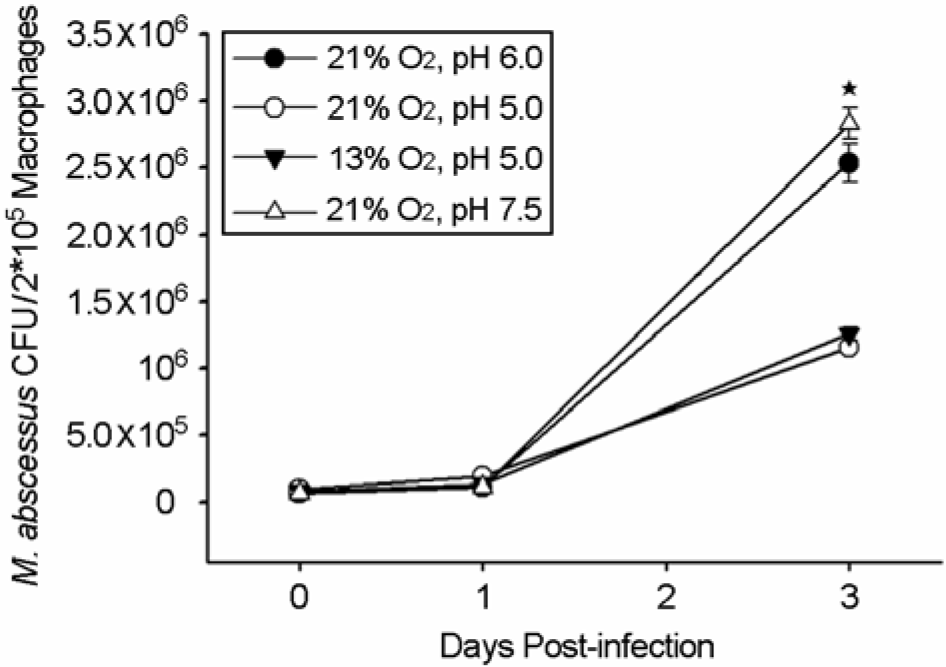 | Figure 3.Growth of M. abscessus conditional variants strains in BMDM. M. abscessus # 1 (•) and # 4 (Δ) survive and more actively multiply in BMDMs than M. abscessus # 2 (○) and # 3 (▾). BMDMs were infected with 2.5×105 M. abscessus strains for 4 h. Bacterial cfu in cell lysates was determined at the indicated intervals. Data are the mean of duplicated determinations of two separate experiments. Data are presented as the mean ± SD of two experiments. Significant differences (∗p<0.05) compared with conditional morphotype variants are indicated. M. abscessus strains were grown under each indicated condition as # 1 (•); 21% O2, pH 6.0, # 2 (○); 21% O2, pH 5.0, # 3 (▾); 13% O2, pH 5.0, and # 4 (Δ); 21% O2, pH 7.5. |
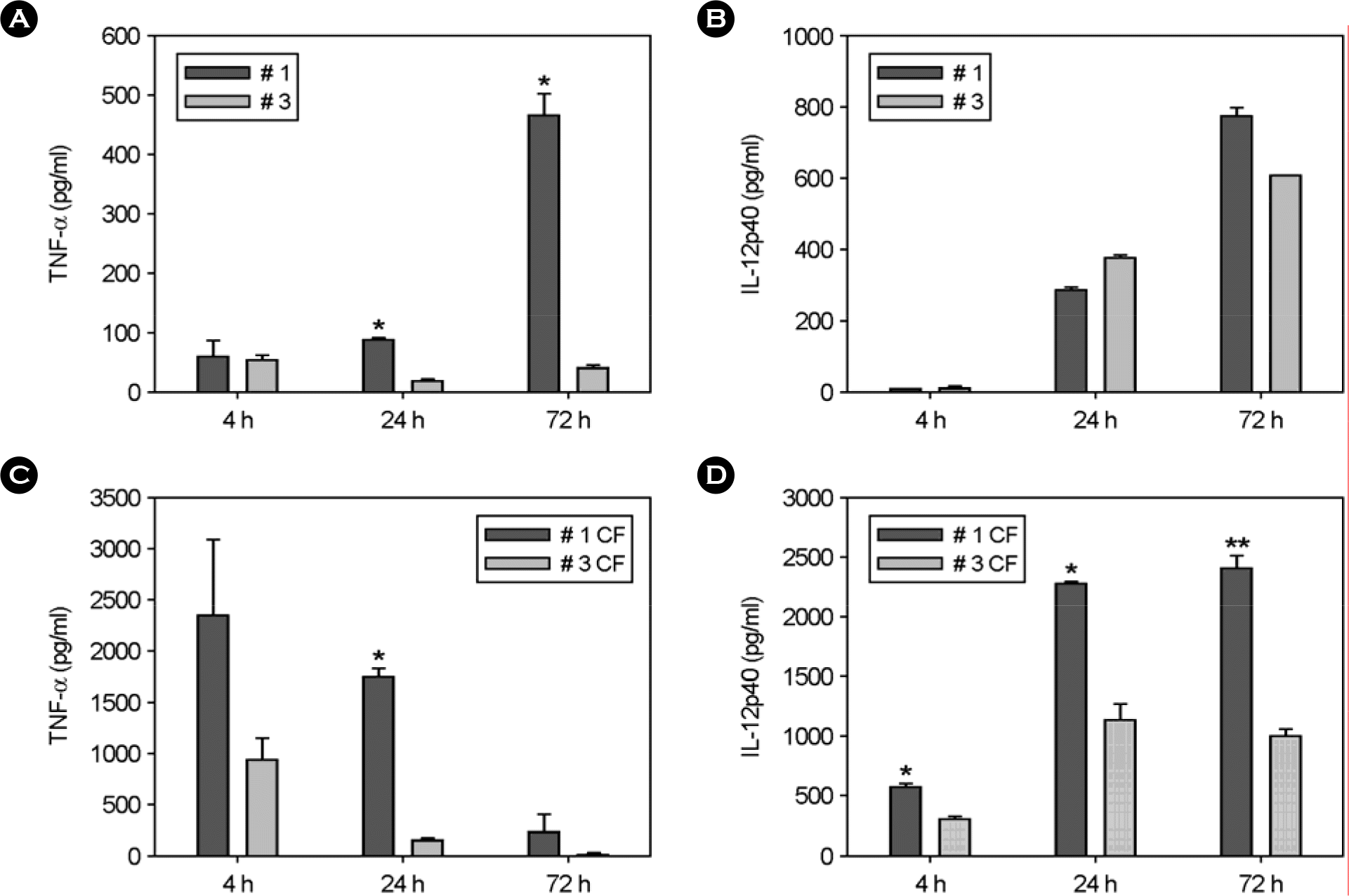 | Figure 4.TNF-α and IL-12p40 production in BMDMs stimulated with M. abscessus conditional variants and treated with culture filtrate (CF). BMDMs were infected for 4 hr with M. abscessus # 1 and # 3 variants at a MOI of 1:1, washed three times, and further incubated for 72 hr (A, B). The CF was used at a concentration of 1 μg/ml (C, D). TNF-α and IL-12p40 were measured in culture supernatants at 4, 24 and 72 hr post-infection. Data are presented as the mean ± SD of three experiments. Significant differences (∗p<0.05; ∗∗p<0.01) compared with strain or CF of smooth variant are indicated. |
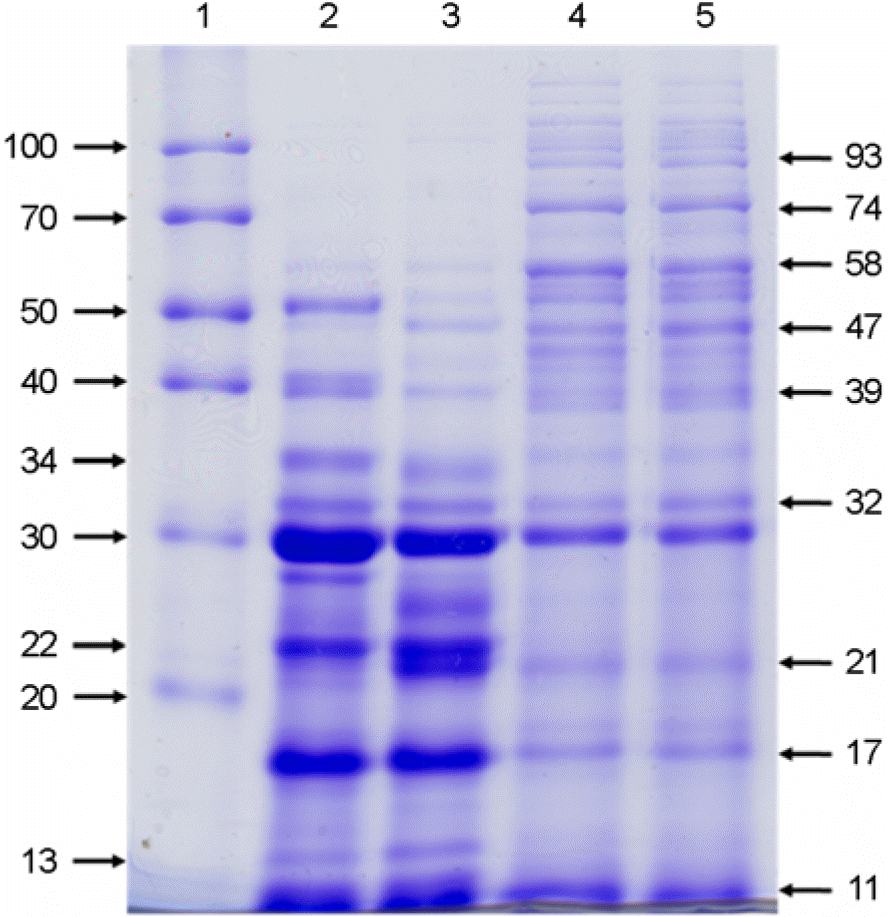 | Figure 5.Comparison of the culture filtrate (CF) protein profiles from M. abscessus variants grown under 21% O2, pH 6.0 (2), 21% O2, pH 7.5 (3), 21% O2, pH 5.0 (4), 13% O2, pH 5.0 (5) conditions. The first lane (1) was loaded with protein molecular weight marker. The CF proteins (30 μg of total proteins for each lane) were separated by 12% SDS-PAGE. The gel was stained with 0.25% Coomassie brilliant blue R250. |
Table 1.
Comparison of differentially expressed proteins under variable culture conditions
| Protein bands (kDa) | ∗Compared with mWR-pH 6.0 (21%), change of protein expression in; | ||
|---|---|---|---|
| pH 7.5 (21%) | pH 5.0 (13%) | pH 5.0 (21%) | |
| 127 | – | ↑ | ↑ |
| 114 | – | ↑ | ↑ |
| 101 | – | ↑ | ↑ |
| 93 | – | ↑ | ↑ |
| 74 | – | ↑ | ↑ |
| 58 | – | ↑ | ↑ |
| 55 | – | ↑ | ↑ |
| 52 | ↑ | ↓ | ↓ |
| 48 | ↑ | – | – |
| 47 | – | ↑ | ↑ |
| 44 | – | ↑ | ↑ |
| 41 | ↓ | ↓ | ↓ |
| 39 | ↓ | ↓ | ↓ |
| 38 | – | ↑ | ↑ |
| 34 | ↓ | ↓ | ↓ |
| 33 | ↑ | – | – |
| 31 | – | ↓ | ↓ |
| 30 | – | ↓ | ↓ |
| 28 | ↓ | ↓ | ↓ |
| 25 | ↑ | – | – |
| 22 | – | ↓ | ↓ |
| 21 | ↑ | ↑ | ↑ |
| 20 | ↓ | ↓ | ↓ |
| 18 | – | – | – |
| 17 | – | ↓ | ↓ |
| 13 | ↑ | ↓ | ↓ |
| 11 | – | ↓ | ↓ |
∗ Arrows indicate the expression levels of M. abscessus extracellular-proteins cultivated in each culture condition compared with those produced from normal condition (21% O2, pH 6.0). The up-arrow (↑), down-arrow (↓), and hyphen (–) represent the increased (or present), decreased (or absent), and same (not significantly different) level of protein expressions to normal culture condition, respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download