Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV), a member of the genus Arterivirus in the family Arteriviridae, is the most important viral pathogens in swine industry worldwide. Here, we have investigated 5′ and 3′ cis-acting RNA elements required for PRRSV genome replication. Using the infectious PRRSV cDNA, we have manipulated the genomic RNA to generate mutant genomic RNAs, transfected these mutants into susceptible MARC-145 cells, and examined the competence of RNA replication. We found three genetic factors that were essential for viral replication. First, the cap structure present at the 5′-end of the genome was absolutely required for RNA replication. Secondly, polyadenylation of the genomic RNA at the 3′-end was also essential for RNA replication. Thirdly, approximately 100-nucleotide region just upstream of the N protein-coding region was crucial for genomic RNA replication. Taken together, our findings indicate that replication of PRRSV genomic RNA requires three important cis-acting RNA elements: 5′ cap structure, 3′ poly(A) motif, and an internal sequence of about 100 nucleotides. Further investigation is needed to elucidate the molecular mechanism(s) of how these elements act on PRRSV genome replication.
Go to : 
References
1). Andrews NC, Baltimore D. Purification of a terminal uridylyltransferase that acts as host factor in the in vitro poliovirus replicase reaction. Proc Natl Acad Sci USA. 83:221–225. 1986.

2). Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, Christianson WT, Morrison RB, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest. 4:127–133. 1992.

3). Brierley I, Digard P, Inglis SC. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 57:537–547. 1989.

4). Cavanagh D. Nidovirales: a new order comprising Corona-viridae and Arteriviridae. Arch Virol. 142:629–633. 1997.
5). Choi YJ, Yun SI, Kang SY, Lee YM. Identification of 5′ and 3′ cis-acting elements of the porcine reproductive and respiratory syndrome virus: acquisition of novel 5′ AU-rich sequences restored replication of a 5′-proximal 7-nucleotide deletion mutant. J Virol. 80:723–736. 2006.
7). Clyde K, Harris E. RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication. J Virol. 80:2170–2182. 2006.

7). Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Gorcyca DE, Chladek DW. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 4:117–126. 1992.

8). Cui T, Sankar S, Portar AG. Binding of encephalomyo-carditis virus RNA polymerase to the 3′-noncoding region of the viral RNA is specific and requires the 3′-poly(A) tail. J Biol Chem. 286:26093–26098. 1993.

9). Dolja VV, Grama DP, Morozov SY, Atabekov JG. Potato virus X-related single- and double-stranded RNAs: characterization and identification of terminal structures. FEBS Lett. 214:308–312. 1987.
10). Gagnon CA, Dea S. Differentiation between porcine reproductive and respiratory syndrome virus isolates by restriction fragment length polymorphism of their ORFs 6 and 7 genes. Can J Vet Res. 62:110–116. 1998.
11). Hofmann MA, Brian DA. The 5′ end of coronavirus minus-strand RNAs contains a short poly(U) tract. J Virol. 65:6331–6333. 1991.

12). Jupin I, Bouzoubaa S, Richards K, Jonard G, Guiley H. Multiplication of beet necrotic yellow vein virus RNA 3 lacking a 3′ poly(A) tail is accompanied by reappearance of the poly(A) tail and novel short U-rich tract preceding it. Virology. 178:281–284. 1990.
13). Kang SY, Yun SI, Park HS, Park CK, Choi HS, Lee YM. Molecular characterization of PL97–1, the first Korean isolate of the porcine reproductive and respiratory syndrome virus. Virus Res. 104:165–179. 2004.

14). Kwang J, Kim HS, Joo HS. Cloning, expression, and sequence analysis of the ORF4 gene of the porcine reproductive and respiratory syndrome virus MN-1b. J Vet Diagn Invest. 6:293–296. 1994.

15). Lin YJ, Liao CL, Lai MMC. Identification of the cis-acting signal for minus-strand RNA synthesis of a murine corona-virus: Implications for the role of minus-strand RNA in RNA replication and transcription. J Virol. 68:8131–8140. 1994.

16). Mardassi H, Mounir S, Dea S. Identification of major differences in the nucleocapsid protein genes of a Quebec strain and European strains of porcine reproductive and respiratory syndrome virus. J Gen Virol. 75:681–685. 1994.

17). Meng XJ, Paul PS, Halbur PG. Molecular cloning and nucleotide sequencing of the 3-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 75:1795–1801. 1994.

18). Meng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the USA and Europe. Arch Virol. 140:745–755. 1995.

19). Meulenberg JJM, Hulst MM, de Meijer EJ, Moonen PLJM, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJM. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 192:62–72. 1993.

20). Morozov I, Meng XJ, Paul PS. Sequence analysis of open reading frames (ORFs) 2–4 of a US isolate of porcine reproductive and respiratory syndrome virus. Arch Virol. 140:1313–1319. 1995.
21). Murtaugh MP, Elam MR, Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 140:1451–1460. 1995.

22). Nagy PD, Carpenter CD, Simon AE. A novel 3′-end repair mechanism in an RNA virus. Proc Natl Acad Sci USA. 94:1113–1118. 1997.

23). Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 73:270–280. 1999.

24). Pasternak AO, Spaan WJM, Snijder EJ. Nidovirus transcription: how to make sense…? J Gen Virol. 87:1403–1421.

25). Sagripanti JL, Zandomeni RO, Weinmann R. The cap structure of simian hemorrhagic fever virion RNA. Virology. 151:146–150. 1986.

26). Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed.Cold Spring Harbor Laboratory;Cold Spring Harbor, NY: 1989.
27). Sangker S, Proter AG. Expression, purification, and properties of recombinant encephalomyocarditis virus RNA-dependent RNA polymerase. J Virol. 65:2993–3000. 1991.

28). Sawicki DL, Gomatos PJ. Replication of semliki forest virus: Polyadenylate in plus-strand RNA and polyuridylate in minus-strand RNA. J Virol. 20:446–464. 1976.

29). Snijder EJ, Meulenberg JJM. The molecular biology of arteriviruses. J Gen Virol. 79:961–979. 1998.

30). Snijder EJ, van Tol H, Pedersen KW, Raamsman MJ, de Vries AA. Identification of a novel structural protein of arteri-viruses. J Virol. 73:6335–6345. 1999.

31). Spector DH, Baltimore D. Polyadenylic acid on poliovirus RNA. IV. Poly(U) in replicative intermediate and double-stranded RNA. Virology. 67:498–505. 1975.
32). Wensvoort G. Lelystad virus and the porcine epidemic abortion and respiratory syndrome. Vet Res. 24:117–124. 1993.
33). Wensvoort G, Terpstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, Broekhuijsen JM, Moonen PLJM, Zetstra T, de Boer EA, Tibben HJ, de Jong MF, vant Veld P, Groenland GJR, van Gennep JA, Voets MT, Verheijden JHM, Braamskamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet Q. 13:121–130. 1991.

Go to : 
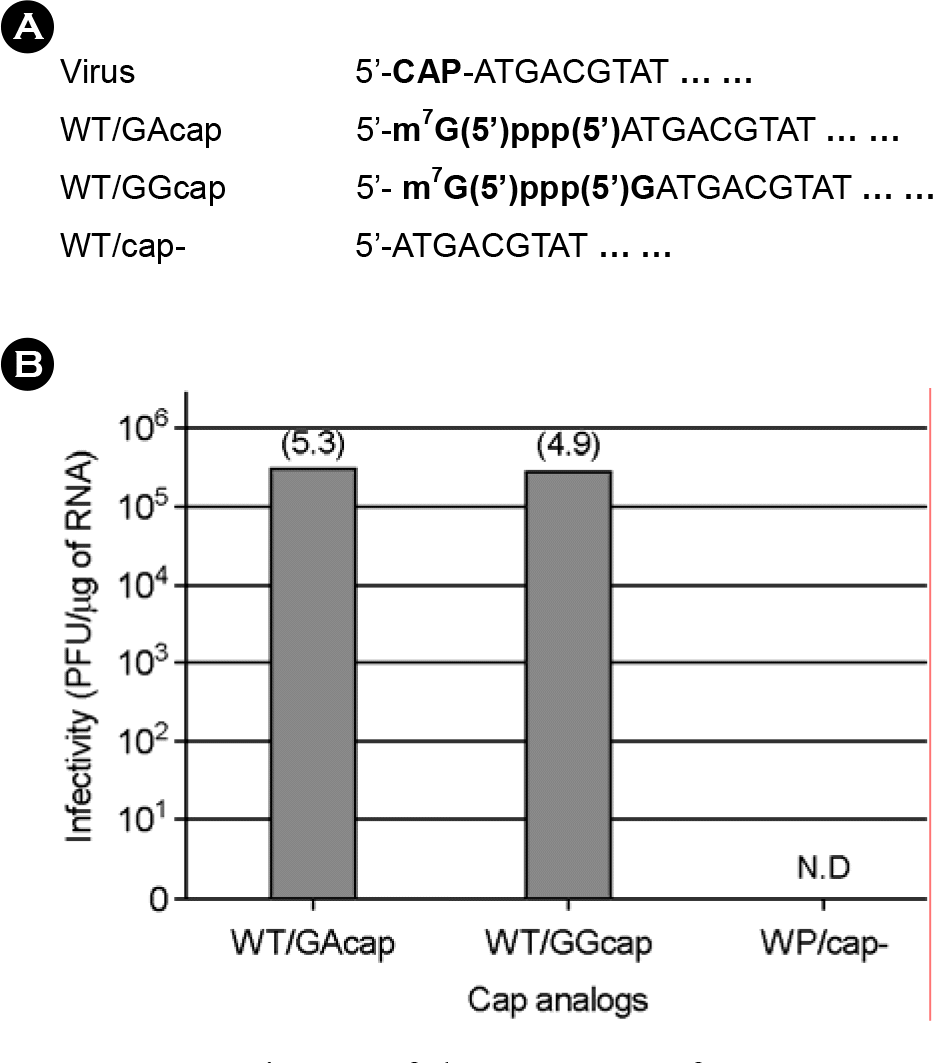 | Figure 1.Requirement of the cap structure for PRRSV RNA replication. (A) Schematic presentation of the 5′-end region of three synthetic RNA transcripts derived from WT/GAcap, WT/GGcap, and WT/cap- cDNAs. (B) Determination of the specific infectivity of synthetic RNAs transcribed from the corresponding constructs. N.D, not detected. |
 | Figure 2.Requirement of the poly(A) tract for PRRSV RNA replication. (A) Schematic presentation of the 3′-end region of WT and Mutant/NPA that does not contain the poly(A) tail. (B) Determination of the specific infectivity of synthetic RNAs transcribed from WT and Mutant/NPA constructs. N.D, not detected. |
 | Figure 3.Schematic depiction of luciferase-expressing PRRSV viral replicons. The five viral replicons Mutant/nt14500, Mutant/nt14600, Mutant/nt14700, Mutant/nt14800, and Mutant/nt14900 have large internal deletions of nt 12163∼14500, nt 12163∼14600, nt 12163∼14700, nt 12163∼14800, and nt 12163∼14900, respectively. An expression cassette consisting of the EMCV IRES-driven LUC gene was inserted at the deletion site to facilitate the monitoring of viral replication. |
 | Figure 4.Expression of LUC gene. Naïve BHK-21 cells were transfected with the PRRSV RNAs transcribed from each cDNA template and seeded on 6-well plates at a density of 4×105 cells per well. After 36 hr incubation, cell lysates were prepared for LUC assays. The experiments were performed in duplicate; mean values are shown. |
Table 1.
Oligonucleotides used for mutagenesis in this study.
| Oligo-nucleotides | Sequencea | Polarity |
|---|---|---|
| PR-F | 5′-TCCCGGCCAAAGCTTCATGATTTT | sense |
| PRnpa-R | 5′-AGGGCGGCCGCAACGTCTAGAATTTCGGCCGCATGGTT | antisense |
| PR14600-F | 5′-GCAGACGCGTGGAGCAGTAGTTGCACTCCTTTGG | sense |
| PR14700-F | 5′-GCAGACGCGTTTCTGGCCCCTGCCCACCACGTTG | sense |
| PR14800-F | 5′-GCAGACGCGTGGTCAACGGCACATTGGTGCCCGG | sense |
| PR14900-F | 5′-GCAGACGCGTCAACGGCAAGCAGCAGAAGAGAAA | sense |
| PR-R | 5′-CCTGCAGGGCGGCCGCAACGTTTTTTTTTTTTTT | antisense |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download