Abstract
Retinal pigment epithelium (RPE) constituting the outer blood-retina barrier plays an important role in ocular defense mechanism. Many studies reported that RPE participates in ongoing immune responses in the retina. However, the exact mechanism is still uncertain. Toll-like receptors (TLRs) participate in the recognition of pathogen-associated molecular patterns (PAMP), such as LPS, zymosan, lipoprotein, and dsRNA. The expression and function of TLRs in human RPE have not been established. In this study, we investigated TLRs expression in human fetal RPE and their recognition of PAMP to determine how human RPE participates in ocular defense mechanism against microbial component. RT-PCR and real time PCR revealed that TLR1 through 5 were constitutively expressed in human fetal RPE, and their expressions were slightly increased by LPS. We determined the TNF-α, IL-6, and IL-8 expression in human fetal RPE after treatment with LPS, zymosan, petidoglycan, or poly I:C. RT-PCR demonstrated that LPS and poly I:C treatment increased the production of TNF-α, IL-6, and IL-8 in human fetal RPE. LPS showed more potent effects on TNF-α and IL-8 production. Peptidoglycan and zymosan did not induce the production of TNF-α. CD14, the co-receptor of LPS was weakly expressed and functioned in recognizing LPS in human fetal RPE. These results suggest that human RPE may participate in ocular defense mechanism against microbial component through toll-like receptors.
Go to : 
References
3). Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipo-proteins through toll-like receptors. Science. 285:732–736. 1999.

4). Chang JH, McCluskey P, Wakefield D. Expression of tolllike receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Invest Ophthalmol Vis Sci. 45:1871–1878. 2004.

5). Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 90:103–108. 2006.

6). Detrick B, Rhame J, Wang Y, Nagineni CN, Hooks JJ. Cytomegalovirus replication in human retinal pigment epithelial cells. Altered expression of viral early proteins. Invest Ophthalmol Vis Sci. 37:814–825. 1996.
7). Elner VM, Elner SG, Bian ZM, Kindezelskii AL, Yoshida A, Petty HR. RPE CD14 immunohistochemical, genetic, and functional expression. Exp Eye Res. 76:321–331. 2003.

8). Elner SG, Petty HR, Elner VM, Yoshida A, Bian ZM, Yang D, Kindezelskii AL. TLR4 mediates human retinal pigment epithelial endotoxin binding and cytokine expression. Trans Am Ophthalmol Soc. 103:126–135. 2005.

9). Elner VM, Scales W, Elner SG, Danforth J, Kunkel SL, Strieter RM. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 54:361–368. 1992.

10). Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 197:1107–1117. 2003.

11). Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 278:8869–8872. 2003.

12). Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 20:29–48. 2001.

13). Janeway CA Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 10:349–350. 1998.
14). Kindzelskii AL, Elner VM, Elner SG, Yang D, Hughes BA, Petty HR. Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments. J Gen Physiol. 124:139–149. 2004.

15). Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 153:7–15. 2004.

16). Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 274:33419–33425. 1999.

17). Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: A link between Toll-like receptor, interferon and viruses. Microbiol Immunol. 48:147–154. 2004.

18). Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. 1997.

19). Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 164:5998–6004. 2000.

20). Nagineni CN, Kutty RK, Detrick B, Hooks JJ. Inflammatory cytokines induce intercellular adhesion molecule-1 (ICAM-1) mRNA synthesis and protein secretion by human retinal pigment epithelial cell cultures. Cytokine. 8:622–630. 1996.

21). Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the tolllike receptor-4 complex. J Immunol. 164:558–561. 2000.

22). Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 16:1–14. 2005.

23). Song PI, Abraham TA, Park Y, Zivony AS, Harten B, Edelhauser HF, Ward SL, Armstrong CA, Ansel JC. The expression of functional LPS receptor proteins CD14 and Toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 42:2867–2877. 2001.
26). Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 11:19–22. 1999.

27). Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 42:392–403. 1969.

28). Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 31:291–306. 1987.

29). Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 168:554–561. 2002.

30). Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, Pawar H, Yashar BM, Moroi SE, Lichter PR, Petty HR, Richards JE, Abecasis GR, Elner VM, Swaroop A. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 14:1449–1455. 2005.

Go to : 
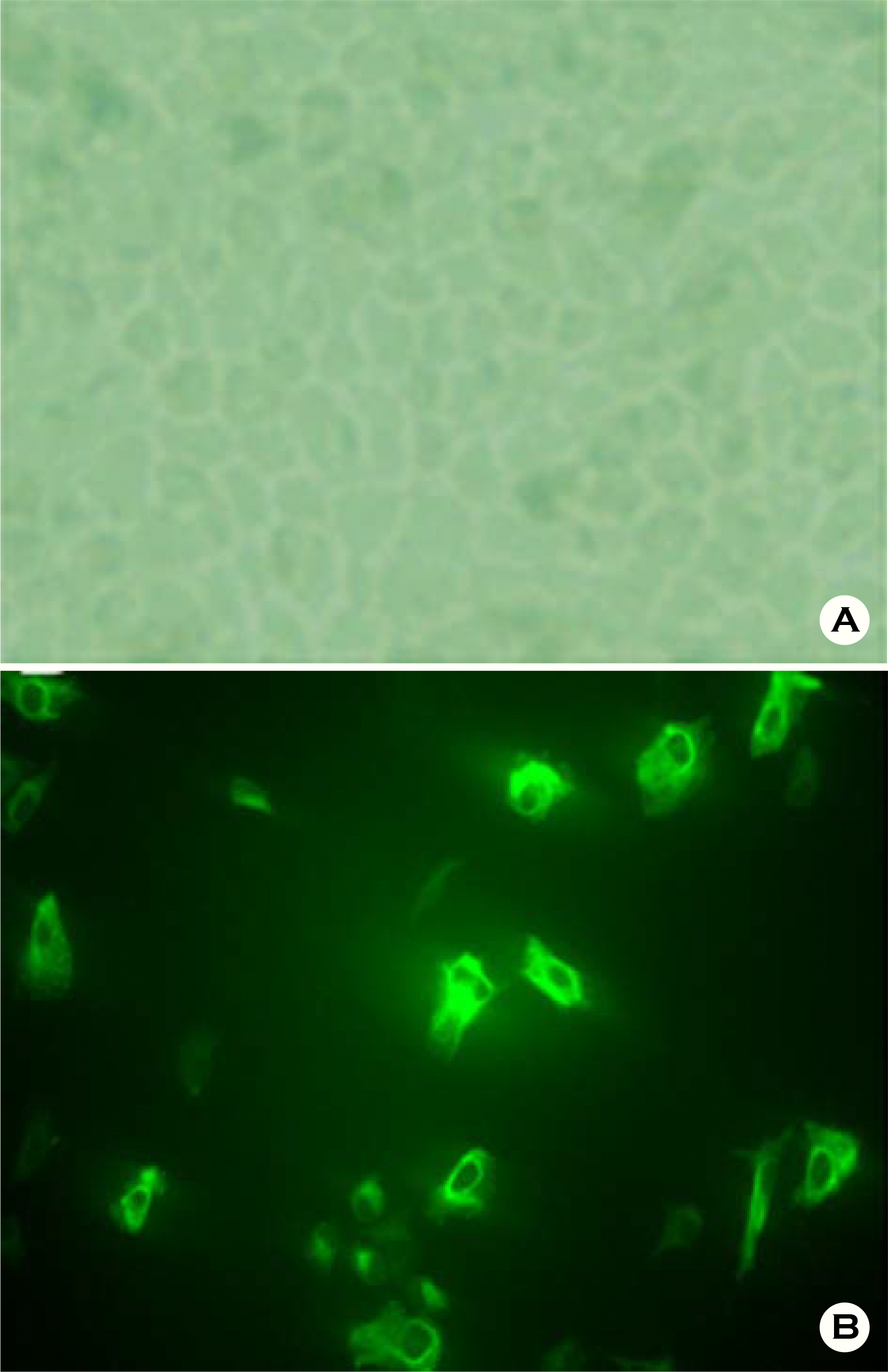 | Figure 1.Culture of human fetal RPE. (A) Phase contrast microscope ×100 (B) Indirect immunostaining of pan-cytokeratin in primarily cultured human fetal RPE. |
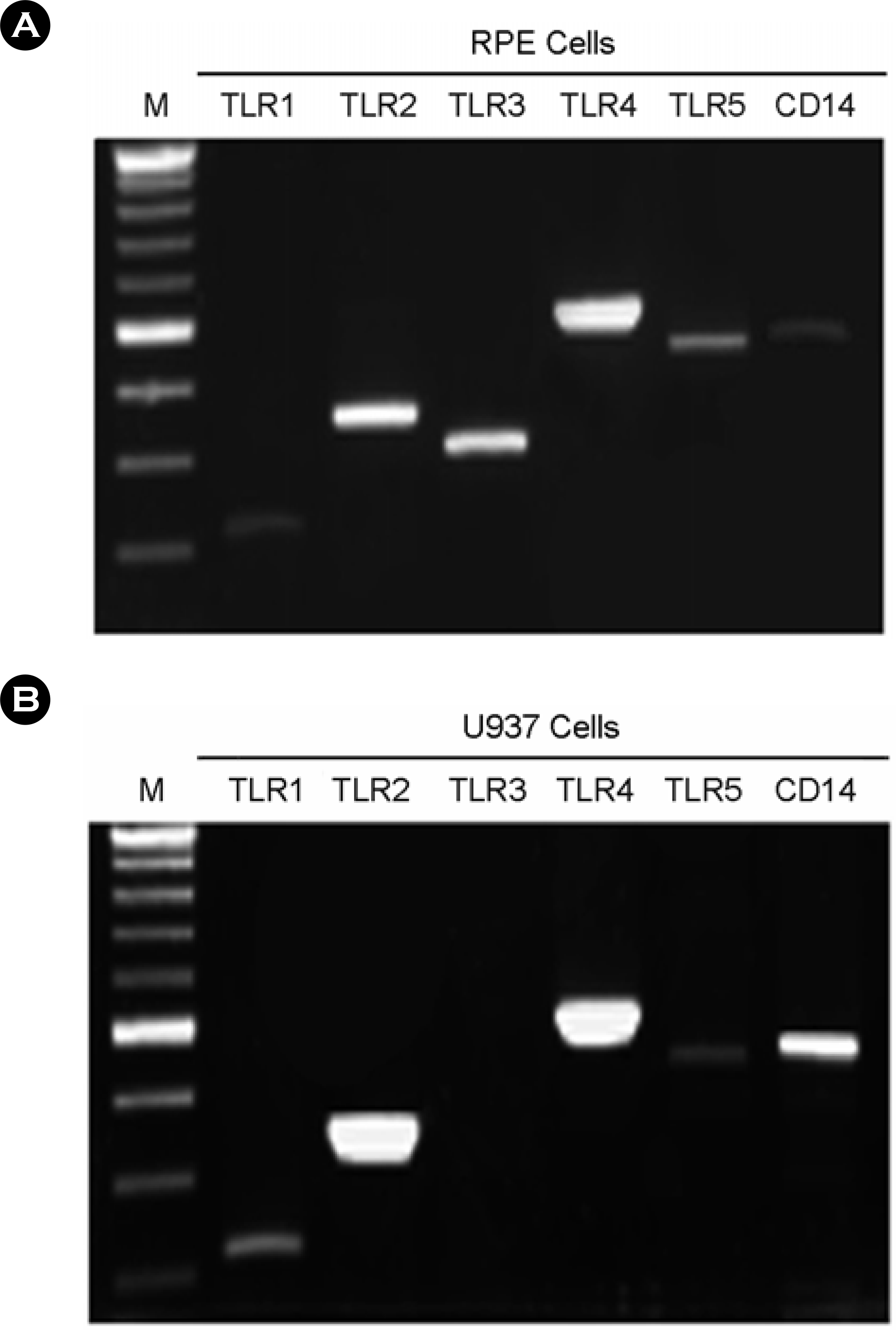 | Figure 2.The expression of TLR1, TLR2, TLR3, TLR4, TLR5, and CD14 in human fetal RPE and U937. (A) The human fetal RPE were cultured in DMEM containing 10% serum. Total RNA was isolated and RT-PCR was performed. (B) The U937 cells were cultured in RPMI 1640 containing 10% serum. Total RNA was isolated and RT-PCR was performed. |
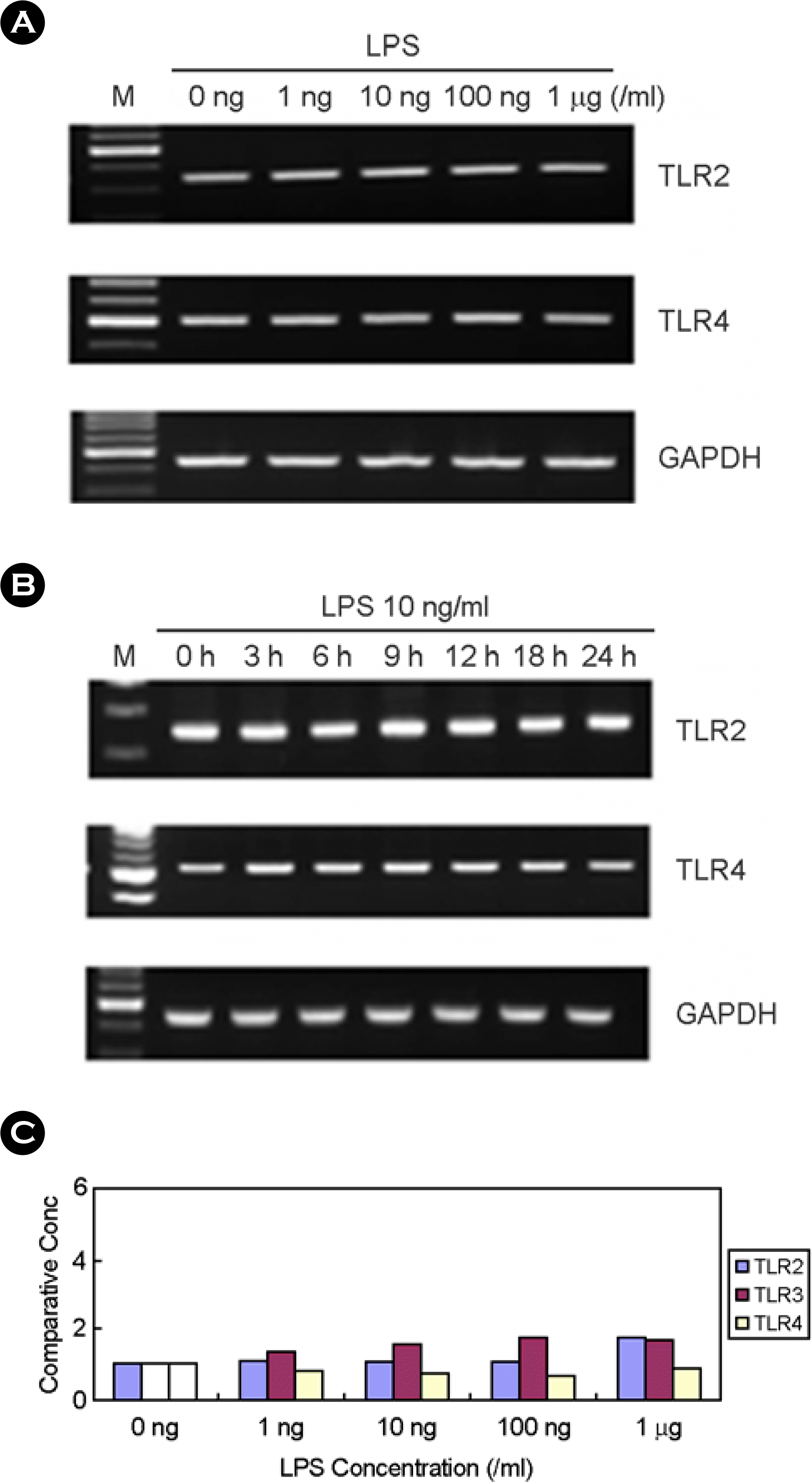 | Figure 3.The effect of LPS on TLR2, TLR3, and TLR4 expression in human fetal RPE. (A) After LPS treatment for 7h at variable dosages, total RNA was isolated and RT-PCR was performed. (B) After treatment with 10 ng/ml of LPS, total RNA was isolated and RT-PCR was performed. (C) After LPS treatment for 7h at variable dosages, real time PCR was performed using primers of GAPDH, TLR2, TLR3, and TLR4. Comparative expression levels were calculated. |
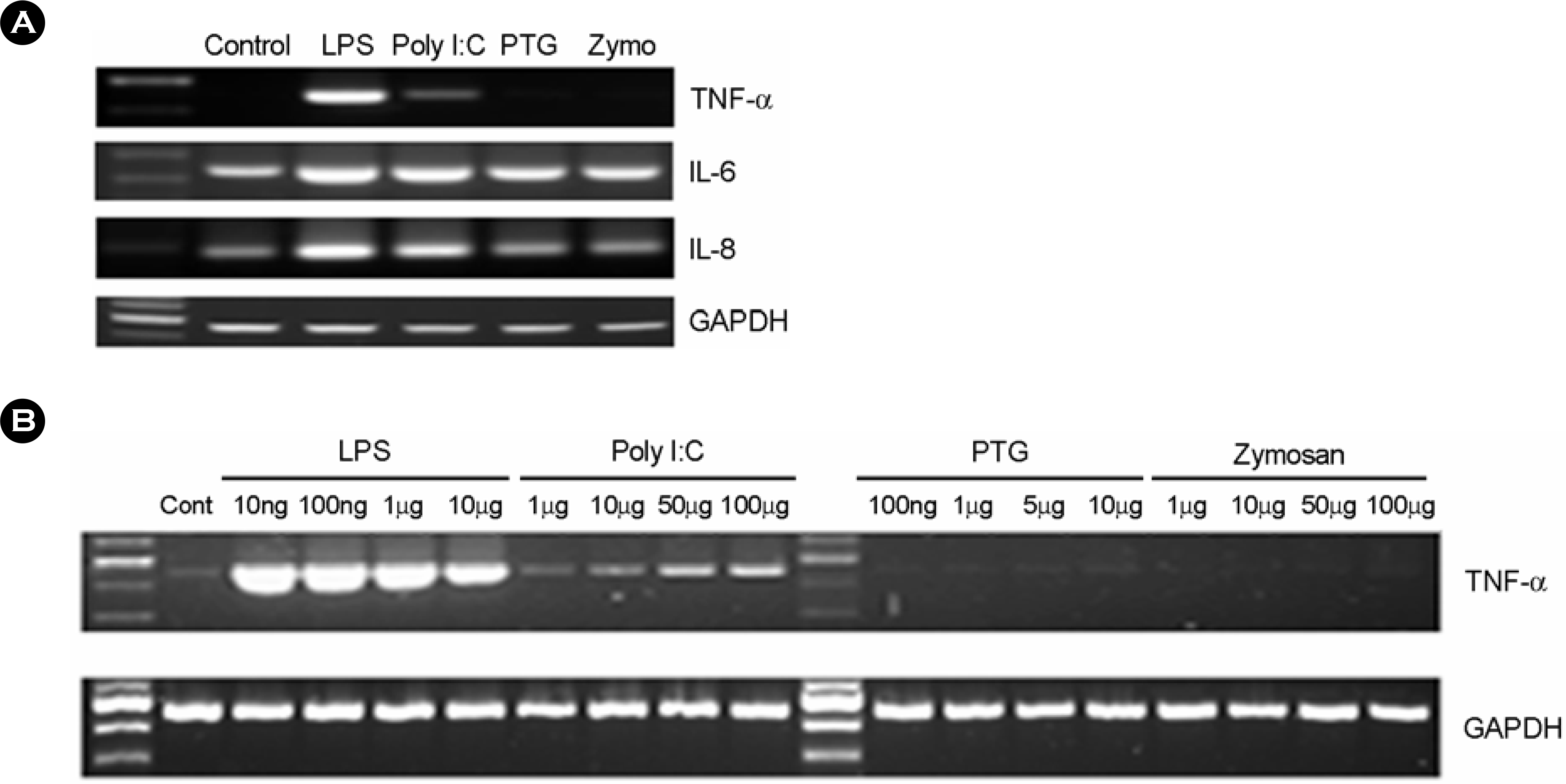 | Figure 4.The effect of microbial components on cytokine expression in human fetal RPE. (A) The human fetal RPE was cultured in the presence or absence of LPS (10 ng/ml), poly I:C (50 μg/ml), peptidoglycan (100 ng/ml), or zymosan (100 μg/ml) for 3h. Total RNA was isolated and mRNA levels of TNF-α, IL-6, and IL-8 were analyzed by RT-PCR. (B) The human fetal RPE was treated with variable dosages of LPS, poly I:C, peptidoglycan, or zymosan for 3h. The levels of TNF-α mRNA were analyzed by RT-PCR. PTG; peptidoglycan, Zymo; zymosan. |
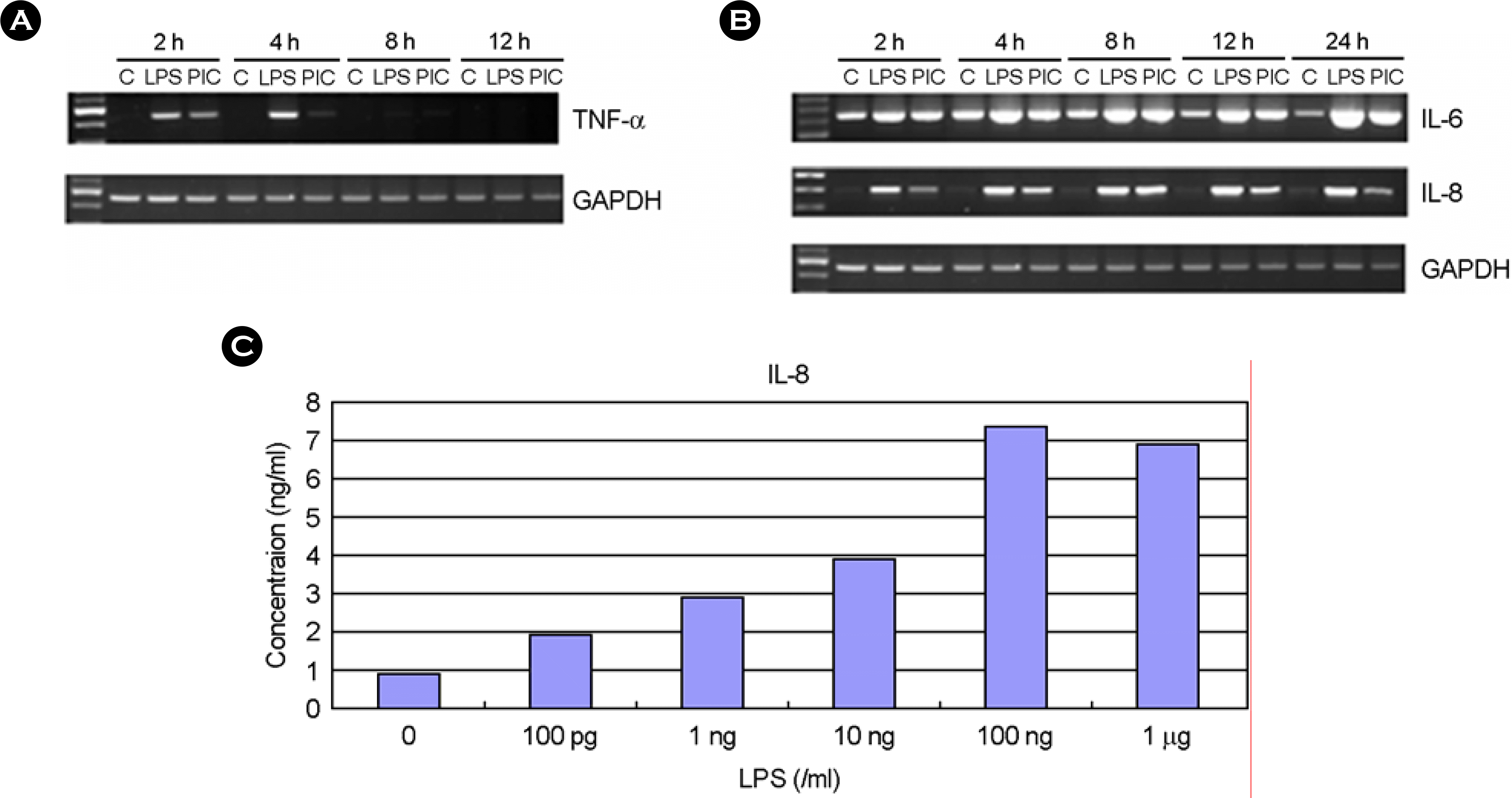 | Figure 5.The effect of LPS and poly I:C on TNF-α, IL-6, IL-8 expression. The human fetal RPE was incubated in the presence or absence of LPS (10 ng/ml) or poly I:C (50 ng/ml). At different time interval, total RNAs were isolated. Then, mRNA levels of TNF-α (A), IL-6 and IL-8 (B) were analyzed using RT-PCR. (C) The cultures were incubated with LPS for 24h and culture supernatants were then harvested. The levels of IL-8 in the culture supernatants were measured using ELISA. PIC; poly I:C |
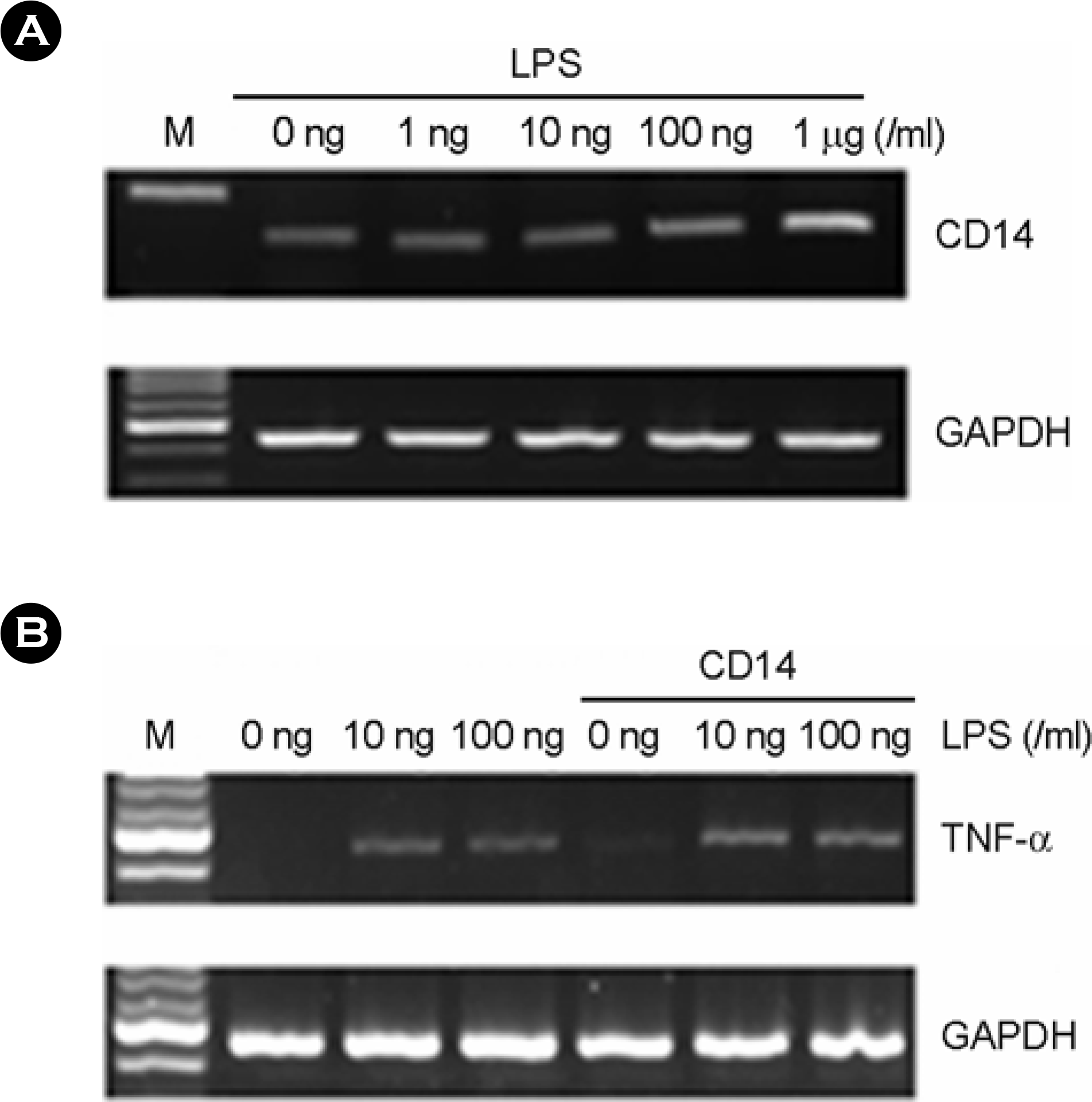 | Figure 6.The effect of serum and soluble CD14 on LPS induced TNF-α expression in human fetal RPE. (A) After LPS treatment for 7h at variable dosages, total RNA was isolated and RT-PCR was performed. (B) The human fetal RPE was cultured in serum-free DMEM. After LPS treatment alone or with soluble CD14 (0.1 ng/ml) for 3h, total RNA was isolated. Then, mRNA levels of TNF-α were analyzed by RT-PCR. |
Table 1.
Primer Sequences for RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download