Abstract
In this study, we investigated the role of toll-like receptor (TLR) and mitogen-activated protein kinase (MAPK) pathways involved in the tumor necrosis factor (TNF)-α and interleukin (IL)-6 expression after stimulation with purified protein derivatives (PPD) or native 38-kDa protein antigen (Ag) of Mycobacterium tuberculosis H37Rv in human primary monocytes. Both PPD and 38-kDa Ag significantly induced TNF-α and IL-6 in human primary monocytes. MAPK [extracellular signal-regulated kinase (ERK) 1/2 and p38] are rapidly phosphorylated in human monocytes stimulated with the PPD or 38-kDa Ag. Both p38 and ERK 1/2 activation are essential for PPD- or 38-kDa-induced TNF-α and IL-6 production. The inhibition of TLR2 and TLR4 by specific antibodies significantly abrogated the 38-kDa-induced secretion of TNF-α and IL-6, whereas blockade of TLR2, but not TLR4, was responsible for the PPD-induced TNF-α and IL-6 production in human monocytes. Collectively, these data suggest that the PPD and 38-kDa Ag differentially interact with TLR2 and TLR4, which in turn mediate an essential role for the early inflammatory immune responses during human tuberculosis.
References
1). 백태현, 권혜숙, 이선, 이지숙, 조은경, 김화중, 이미리 나, 유영춘, 박정규. 결핵균의 TSP 균체항원으로부터 특이항원의 분리 및 특성 분석. J Bact Virol. 34:273–282. 2004.
2). 이지숙, 백태현, 유영춘, 이정림, 신아름, 송창화, 조은 경, 김화중, 박정규. 결핵균 배양여과액으로부터 Ag85 Complex, 38-kDa 및 MTB12 항원의 분리 정제. J Bact Virol. 36:211–221. 2006.
3). Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 62:2536–2544. 1994.
4). Andersen P. The T cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology. 191:537–547. 1994.
5). Blumenthal A, Ehlers S, Ernst M, Flad HD, Reiling N. Control of mycobacterial replication in human macrophages: roles of extracellular signal-regulated kinases 1 and 2 and p38 mitogen-activated protein kinase pathways. Infect Immun. 70:4961–4967. 2002.

6). Bothamley GH, Beck JS, Potts RC, Grange JM, Kardjito T, Ivanyi J. Specificity of antibodies and tuberculin response after occupational exposure to tuberculosis. J Infect Dis. 166:182–186. 1992.

7). Brightbill HD, Libraty DH, Krutzik SR, Yang R-B, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 285:732–736. 1999.

8). Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 280:20961–20967. 2005.
9). Chang Z, Choudhary A, Lathigra R, Quiocho FA. The immunodominant 38-kDa lipoprotein antigen of Mycobacterium tuberculosis is a phosphate-binding protein. J Biol Chem. 269:1956–1958. 1994.
10). Collins HL, Kaufmann SH. The many faces of host responses to tuberculosis. Immunology. 103:1–9. 2001.

11). Denkers EY, Butcher BA, Del Rio L, Kim L. Manipulation of mitogen-activated protein kinase/nuclear factor-kappaB-signaling cascades during intracellular Toxoplasma gondii infection. Immunol Rev. 201:191–205. 2004.
12). Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 20:55–72. 2002.

13). Fonseca DP, Benaissa-Trouw B, van Engelen M, Kraaijeveld CA, Snippe H, Verheul AF. Induction of cell-mediated immunity against Mycobacterium tuberculosis using DNA vaccines encoding cytotoxic and helper T-cell epitopes of the 38-kilodalton protein. Infect Immun. 69:4839–4845. 2001.
14). Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF-α and IFN-gamma in the differentiation of protective granulomas. Eur J Immunol. 27:3182–3190. 1997.
15). Gilleron M, Quesniaux VF, Puzo G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guerin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J Biol Chem. 278:29880–29889. 2003.
16). Harboe M. The significance of proteins actively secreted by Mycobacterium tuberculosis in relation to immunity and complications of mycobacterial diseases. Int J Lepr Other Mycobact Dis. 60:470–476. 1992.
17). Harboe M, Wiker GH. The 38 kDa protein of Mycobacterium tuberculosis: a review. J Infect Dis. 166:874–884. 1992.
18). Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by TLR2 versus TLR4 agonists results in differential gene expression in murine macrophages. Infect Immun. 69:1477–1482. 2001.
19). Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev. 16:637–646. 2003.

20). Jo EK, Park JK, Dockrell HM. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr Opin Infect Dis. 16:205–210. 2003.

21). Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 298:1911–1912. 2002.

22). Jones BW, Heldwein KA, Means TK, Saukkonen JJ, Fenton MJ. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann Rheum Dis. 60(Suppl 3):iii6–12. 2001.
23). Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, Song CH, Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 74:2686–2696. 2006.

24). Keane J, Gershon S, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 345:1098–1104. 2001.
25). Lee JS, Son JW, Jung SB, Kwon YM, Yang CS, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Ex vivo responses for interferon-gamma and proinflammatory cytokine secretion to low-molecular-weight antigen MTB12 of Mycobacterium tuberculosis during human tuberculosis. Scand J Immunol. 64:145–154. 2006.
26). Lee JS, Song CH, Lim JH, Kim HJ, Park JK, Paik TH, Kim CH, Kong SJ, Shon MH, Jung SS, Jo EK. The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 132:443–449. 2003.
27). Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 170:2409–2416. 2003.
28). Means TK, Golenbock DT, Fenton MJ. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 11:219–232. 2000.

29). Means TK, Wang S, Lien E, Yoshimura A, Golenbock D, Fenton M. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 163:3920–3927. 1999.
30). Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. 1998.
31). Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 172:4425–4434. 2004.

32). Rao K. MAP kinase activation in macrophages. J Leukoc Biol. 69:3–10. 2001.
33). Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 23:194–201. 2003.
34). Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 45:491–503. 1994.
35). Underhill D, Ozinsky A, Smith K, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 96:14459–14463. 1999.

36). Weir RE, Black GF, Dockrell HM, Floyd S, Fine PE, Chaguluka SD, Stenson S, King E, Nazareth B, Warndorff DK, Ngwira B, Crampin AC, Mwaungulu L, Sichali L, Jarman E, Donovan L, Blackwell JM. Mycobacterial purified protein derivatives stimulate innate immunity: Malawians show enhanced tumor necrosis factor alpha, interleukin-1beta (IL-1beta), and IL-10 responses compared to those of adolescents in the United Kingdom. Infect Immun. 72:1807–1811. 2004.
37). Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, Akira S, Paik TH, Jo EK. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 9:382–396. 2007.
38). Zhu X, Venkataprasad N, Thangaraj HS, Hill M, Singh M, Ivanyi J, Vordermeier HM. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 158:5921–5926. 1997.
Figure 1.
SDS-PAGE analysis of purified 38-kDa antigen (Ag) of M. tuberculosis culture filtrate. (A) The 38-kDa Ag was purified from unheated culture filtrate of M. tuberculosis H37Rv by ammonium sulfate precipitation and hydrophobic interaction chromatography, and anion exchange chromatography. The antigen was separated by SDS-PAGE and then analyzed by Coomassie blue staining. (B) Purified 38-kDa Ag separated by IEF on pH 4.7∼5.9 IPG strip in the first dimension and 12% SDS-PAGE in the second dimension. The second dimension gel was blotted onto nitrocellulose and immunoblot with rabbit anti-38 kDa polyclonal antibody.
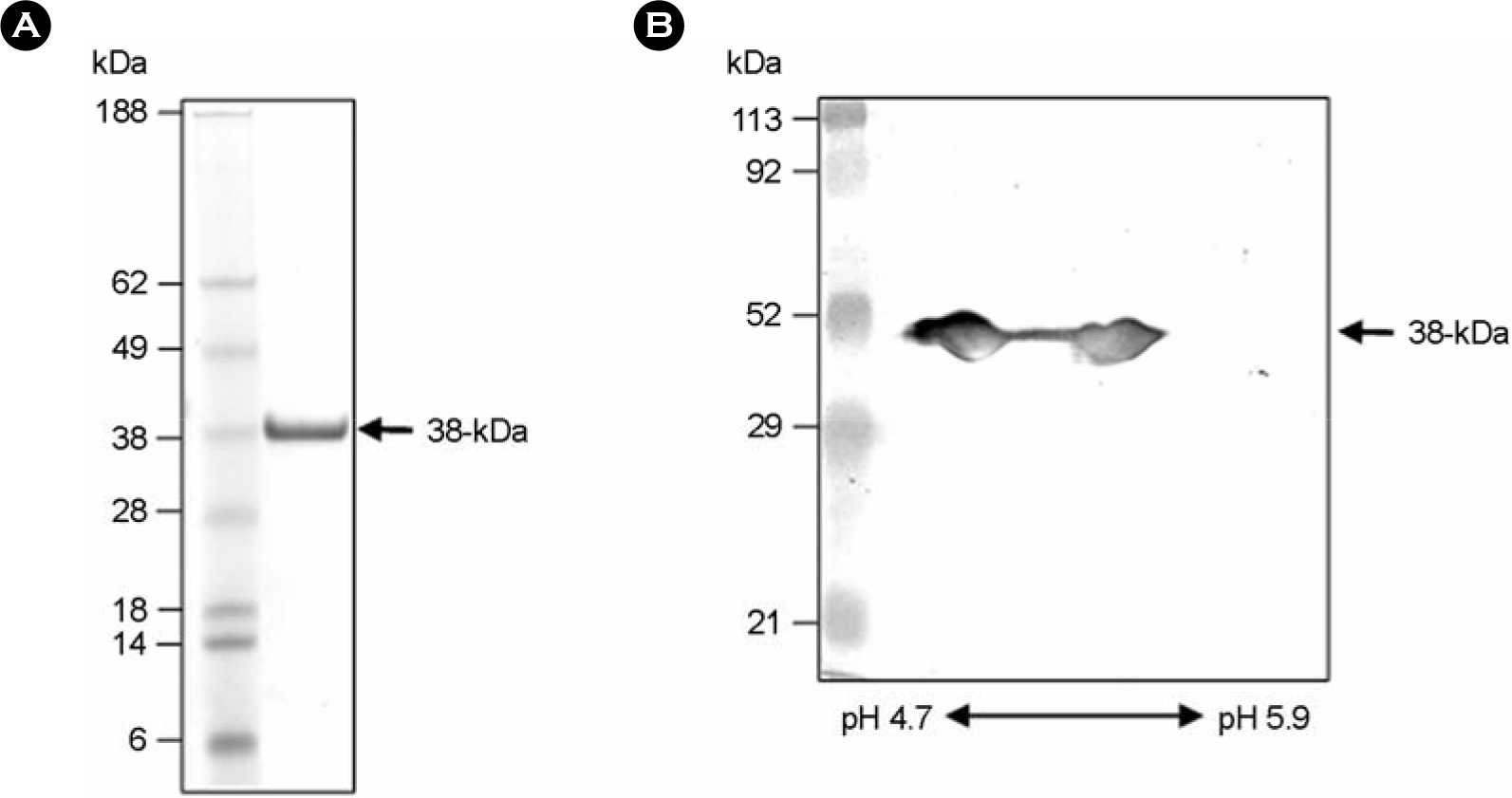
Figure 2.
The PPD or 38-kDa Ag-induced TNF-α and IL-6 secretion in human monocytes. Human monocytes were stimulated with the PPD or 38-kDa Ag (5.0 μg/ml, for each) for 0, 6, 18, 48, and 96 h. The TNF-α (A) and IL-6 (B) production were measured by ELISA. The cytokine synthesis by cells stimulated with the 38-kDa Ag was statistically compared with those by unstimulated control. ∗, p<0.05; ∗∗, p<0.01; ∗∗∗, p<0.001. The error bars indicate SD.
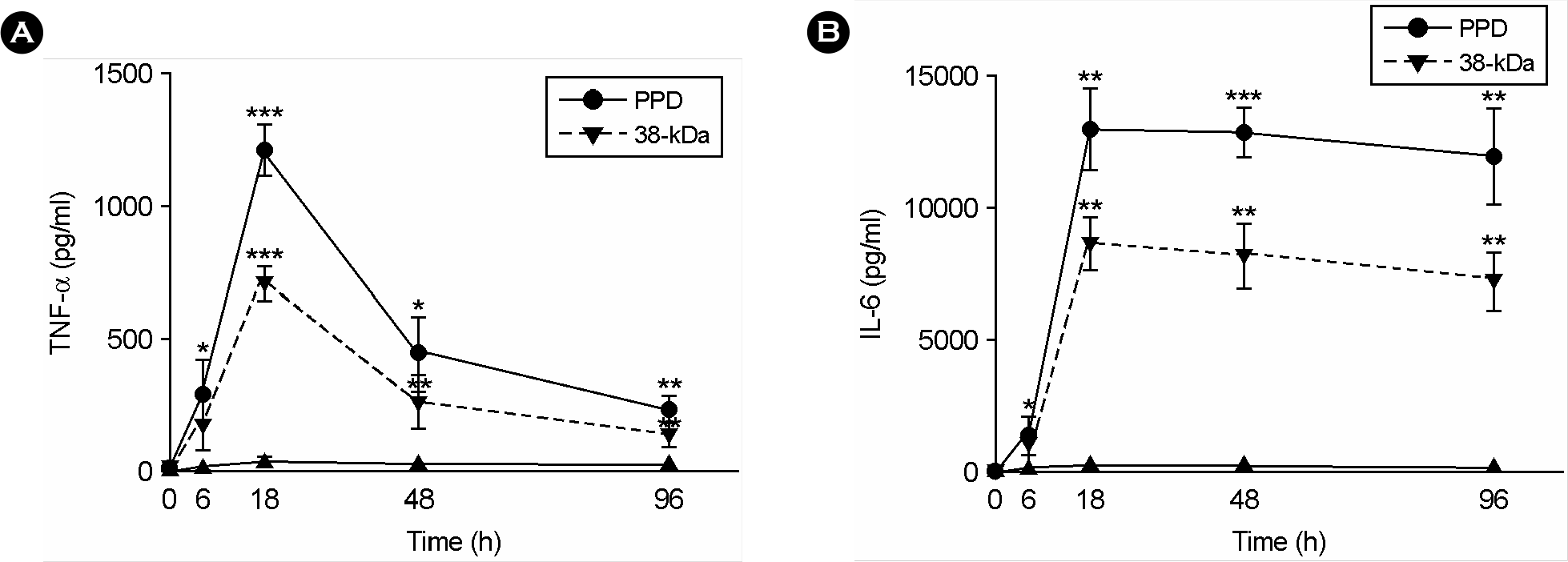
Figure 3.
The PPD or 38-kDa Ag-induced MAPK activation from human monocytes. Human monocytes were stimulated with the PPD (A) or 38-kDa (B) Ag for the times indicated. The cells were lysed, and aliquots of the total cell lysates were separated by SDS-PAGE and immunoblotted as described. The blots were incubated overnight with specific anti-phospho-ERK 1/2, anti-phospho-p38, or control Ab each for the unphosphorylated form, followed by appropriate peroxidase-coupled secondary reagents, and were visualized using ECL. Similar data were obtained in six independent experiments. P, phosphorylated form of Ab; total, total form of Ab.
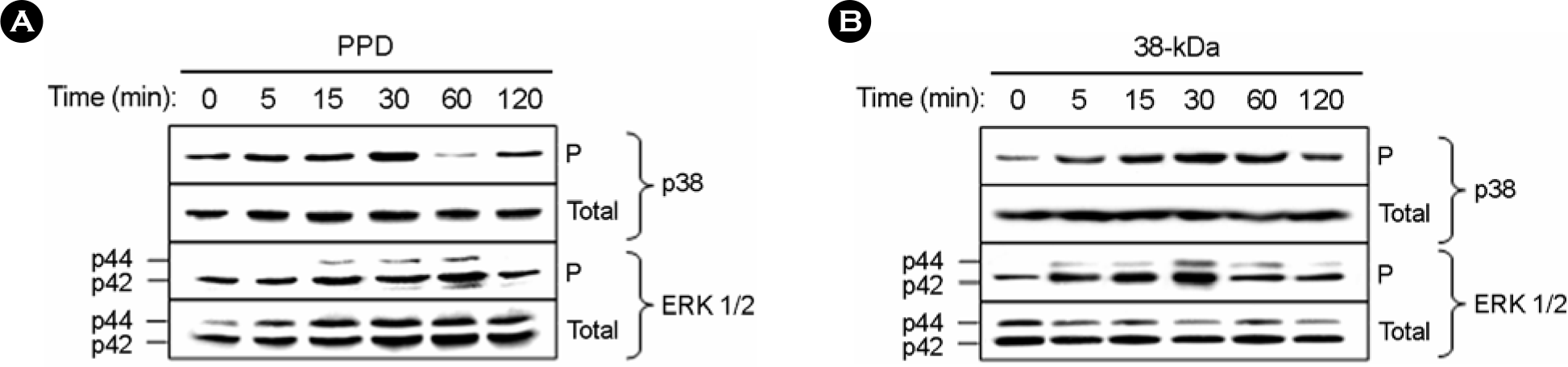
Figure 4.
Effects of MEK or p38 MAPK inhibitors on the PPD or 38-kDa-mediated TNF-α- and IL-6 production. The MEK inhibitor (U0126) or p38 MAPK (SB203580) was added to monocytes at concentrations ranging from 5 to 20 μM at 45 min before stimulation with the PPD (A) or 38-kDa Ag (B) of M. tbc (5.0 μg/ml). The supernatants were harvested after 18 h for cytokine assessment using ELISA. Similar data were obtained in five independent experiments. The mean levels (plus standard errors of the mean) of TNF-α or IL-6 following stimulation with the PPD or 38-kDa Ag were set to 100, and the relative loss of cytokine production in the presence of inhibitor is shown. The solvent control was 0.1% DMSO. SB, SB203580; U, U0126.
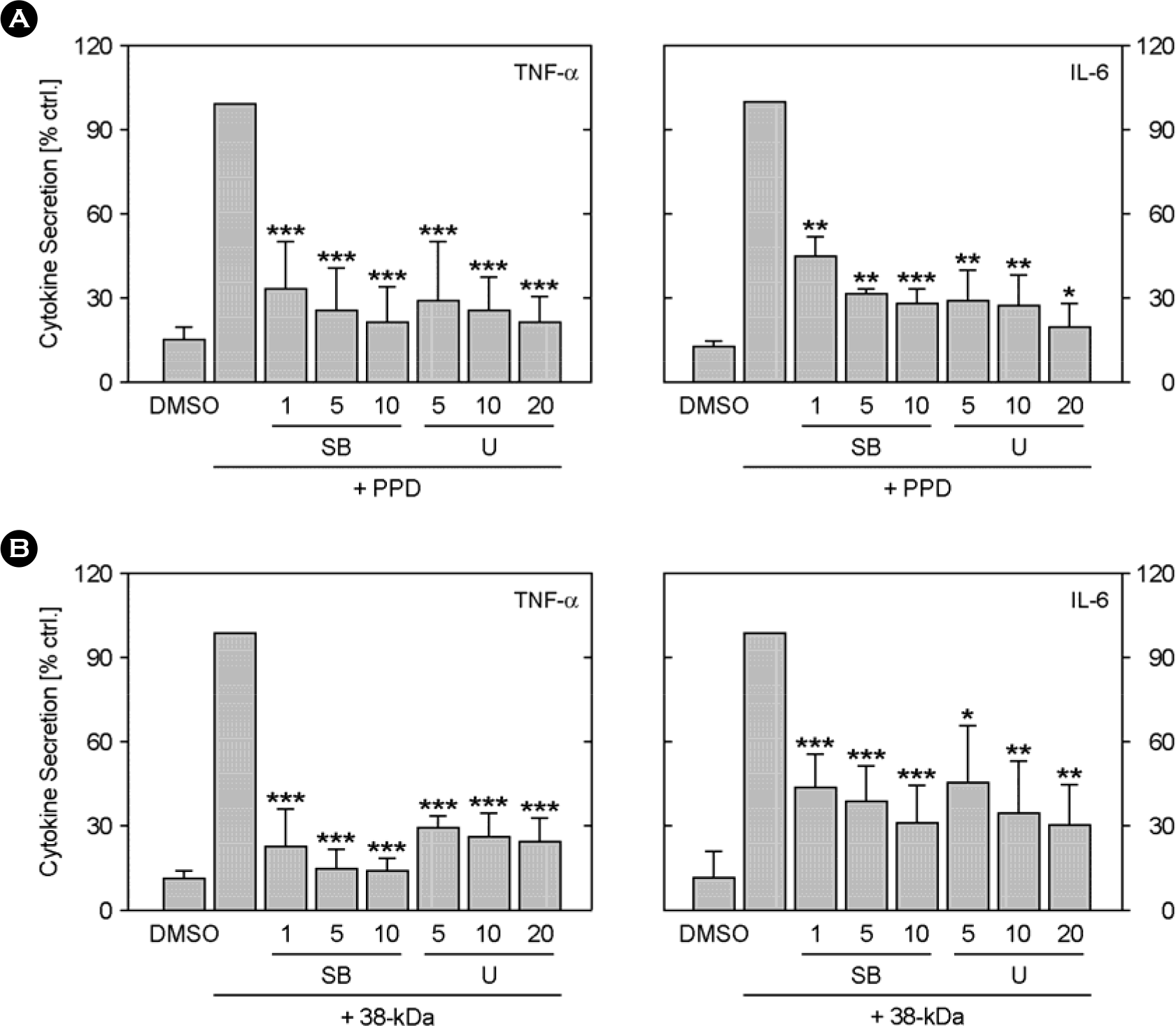
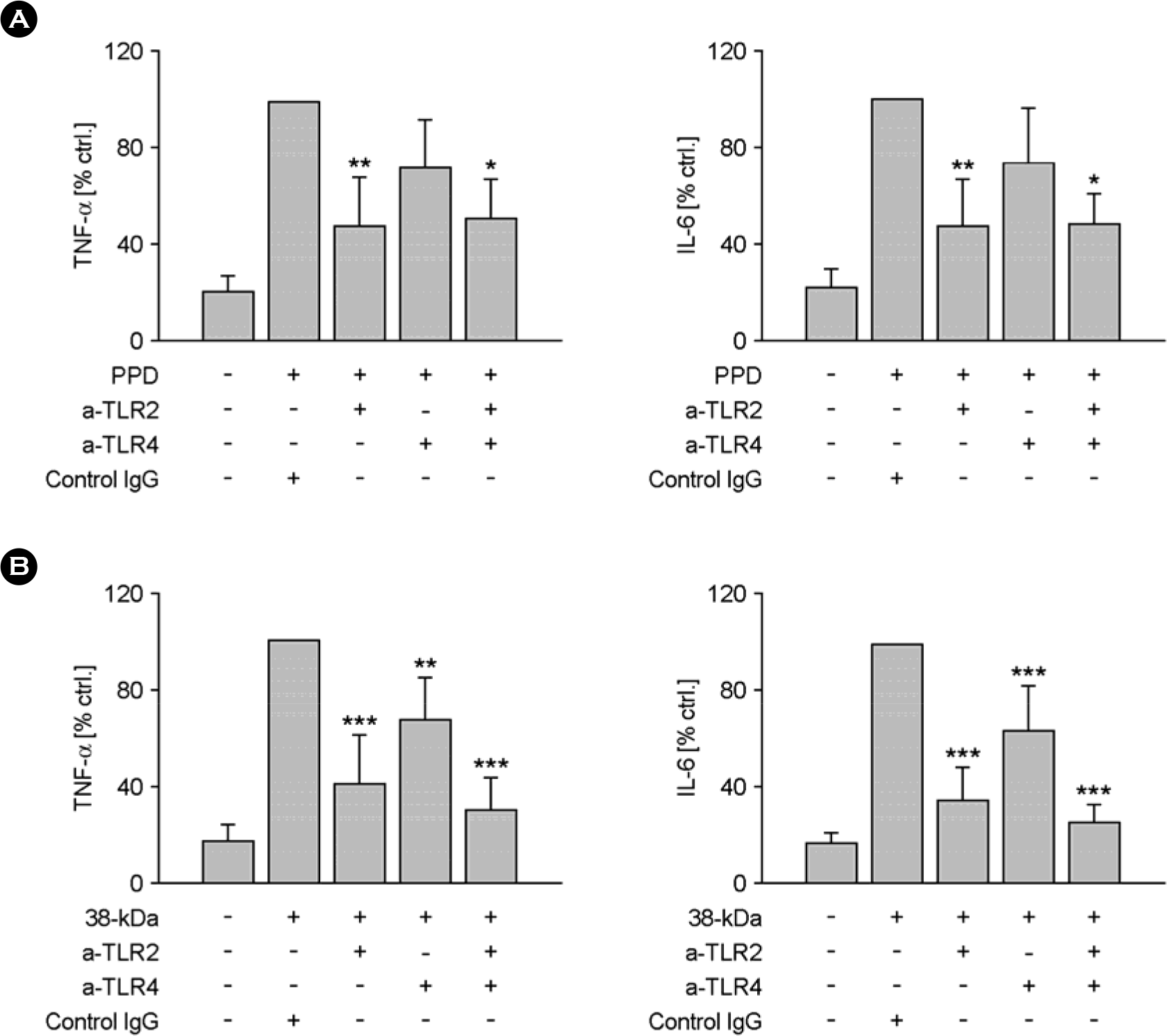
Figure 5.
Effects of anti-TLR2 or anti-TLR4 mAbs on the PPD or 38-kDa-mediated TNF-α and IL-6 production. Human monocytes were preincubated with the indicated amounts of anti-TLR2, anti-TLR4, or isotype control Abs (1 μg/ml) and challenged with the PPD (A) or 38-kDa (B) Ag subsequently for 30 minutes. The supernatants were harvested after 18 h for cytokine assessment using ELISA. Similar data were obtained in five independent experiments. The mean values (plus standard errors of the mean) of TNF-α or IL-6 following stimulation with the 38-kDa Ag were set to 100, and the relative loss of cytokine production in the presence of neutralizing Abs was shown. The solvent control was 0.1% DMSO.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download