Abstract
DNA vaccine approaches have been applied to generate the protective immunity against various pathogens. However, the strength of immune responses induced by DNA vaccine is weak compared with conventional vaccines. The prime-boost vaccination using DNA vaccine and other viral vector has been suggested as one way to circumvent this limitation. In the present study, we used in vivo CTL activity assay to determine CD8+ T cell-mediated immunity induced by prime-boost vaccination with a DNA vaccine (gB498–505 DNA) and recombinant vaccinia virus (VVgB498–505) expressing gB498–505 epitope peptide (SSIEFARL) of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB). The most potent in vivo CTL activity was induced in mice received VVgB498–505 when both gB498–505 and VVgB498–505 were used at priming step and boosted with the alternative vaccine vector expressing whole antigen protein (gBw). Priming with vaccine vector expressing gBw followed by the use of VVgB498–505 at boosting step also induced strong in vivo CTL activity. We also examined in vivo CTL activity after immunization of mice with epitope-expressing vaccine vector at both priming and boosting step. Curiously, in vivo CTL activity mediated by CD8+ T cells was strongly elicited at memory stage when animals were primed with VVgB498–505 and subsequently boosted with gB498–505 DNA. Because the use of VVgB498–505 at priming followed by boosting with gB498–505 DNA induced most optimal immunity, these results suggest that the order of vaccine type should be carefully considered when used vaccine type expressing only epitope for prime-boost vaccination.
Go to : 
References
1). An LL, Whitton JL. A multivalent minigene vaccine, containing B-cell, cytotoxic T-lymphocyte, and Th epitopes from several microbes, induces appropriate responses in vivo and confers protection against more than one pathogen. J Virol. 71:2292–302. 1997.

2). Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 297:2060–2063. 2002.
3). Eo SK, Gierynska M, Kamar AA, Rouse BT. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J Immunol. 166:5473–5479. 2001.

4). Fernando GJ, Khammanivong V, Leggatt GR, Liu WJ, Frazer IH. The number of long-lasting functional memory CD8+ T cells generated depends on the nature of the initial nonspecific stimulation. Eur J Immunol. 32:1541–1549. 2002.

5). Fuller DH, Corb MM, Barnett S, Steimer K, Haynes JR. Enhancement of immunodeficiency virus-specific immune responses in DNA-immunized rhesus macaques. Vaccine. 15:924–926. 1997.

6). Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 18:927–974. 2000.

7). Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 421:852–856. 2003.

8). Kirman JR, Seder RA. DNA vaccination: the answer to stable, protective T-cell memory? Curr Opin Immunol. 15:471–476. 2003.

9). Kursar M, Bonhagen K, Fensterle J, Kohler A, Hurwitz R, Kamradt T, Kaufmann SH, Mittrucker HW. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 196:1585–1592. 2002.

10). Lee S, Gierynska M, Eo SK, Kuklin N, Rouse BT. Influence of DNA encoding cytokines on systemic and mucosal immunity following genetic vaccination against herpes simplex virus. Microbes Infect. 5:571–578. 2003.

11). Lee Y, Eo SK, Rouse RJ, Rouse BT. Influence of CCR7 ligand DNA preexposure on the magnitude and duration of immunity. Virology. 312:169–180. 2003.

12). Letvin NL, Montefiori DC, Yasutomi Y, Perry HC, Davies ME, Lekutis C, Alroy M, Freed DC, Lord CI, Handt LK, Liu MA, Shiver JW. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 94:9378–9383. 1997.

13). Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 252:1662–1667. 1991.

14). Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 195:651–656. 2002.

15). Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci USA. 99:8832–8837. 2002.

16). Park OS, Yoon HA, Aleyas AG, Lee JH, Chae HS, Eo SK. CD8+ T cell-mediated immunity induced by heterologous prime-boost vaccination based on DNA vaccine and recombinant vaccinia virus expressing epitope. Immune Network. 5:89–98. 2005.

17). Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 70:6119–6125. 1996.

18). Randrianarison-Jewtoukoff V, Perricaudet M. Recombinant adenoviruses as vaccines. Biologicals. 23:145–157. 1995.

19). Robinson HL, Montefiori DC, Johnson RP, Manson KH, Kalish ML, Lifson JD, Rizvi TA, Lu S, Hu SL, Mazzara GP, Panicali DL, Herndon JG, Glickman R, Candido MA, Lydy SL, Wyand MS, McClure HM. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 5:526–534. 1999.

20). Schirmbeck R, Stober D, El-Kholy S, Riedl P, Reimann J. The immunodominant, Ld-restricted T cell response to hepatitis B surface antigen (HBsAg) efficiently suppresses T cell priming to multiple Dd-, Kd-, and Kb-restricted HBsAg epitopes. J Immunol. 168:6253–6262. 2002.

21). Sedegah M, Jones TR, Kaur M, Hedstrom R, Hobart P, Tine JA, Hoffman SL. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci U S A. 95:7648–7653. 1998.

22). Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 300:337–339. 2003.

23). Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 170:2540–2548. 2003.
24). Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 300:339–342. 2003.

25). Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 198:889–901. 2003.

26). Wild J, Grusby MJ, Schirmbeck R, Reimann J. Priming MHC-I-restricted cytotoxic T lymphocyte responses to exogenous hepatitis B surface antigen is CD4+ T cell dependent. J Immunol. 163:1880–1887. 1999.
27). Yu Z, Karem KL, Kanangat S, Manickan E, Rouse BT. Protection by minigenes: a novel approach of DNA vaccines. Vaccine. 16:1660–1667. 1998.
Go to : 
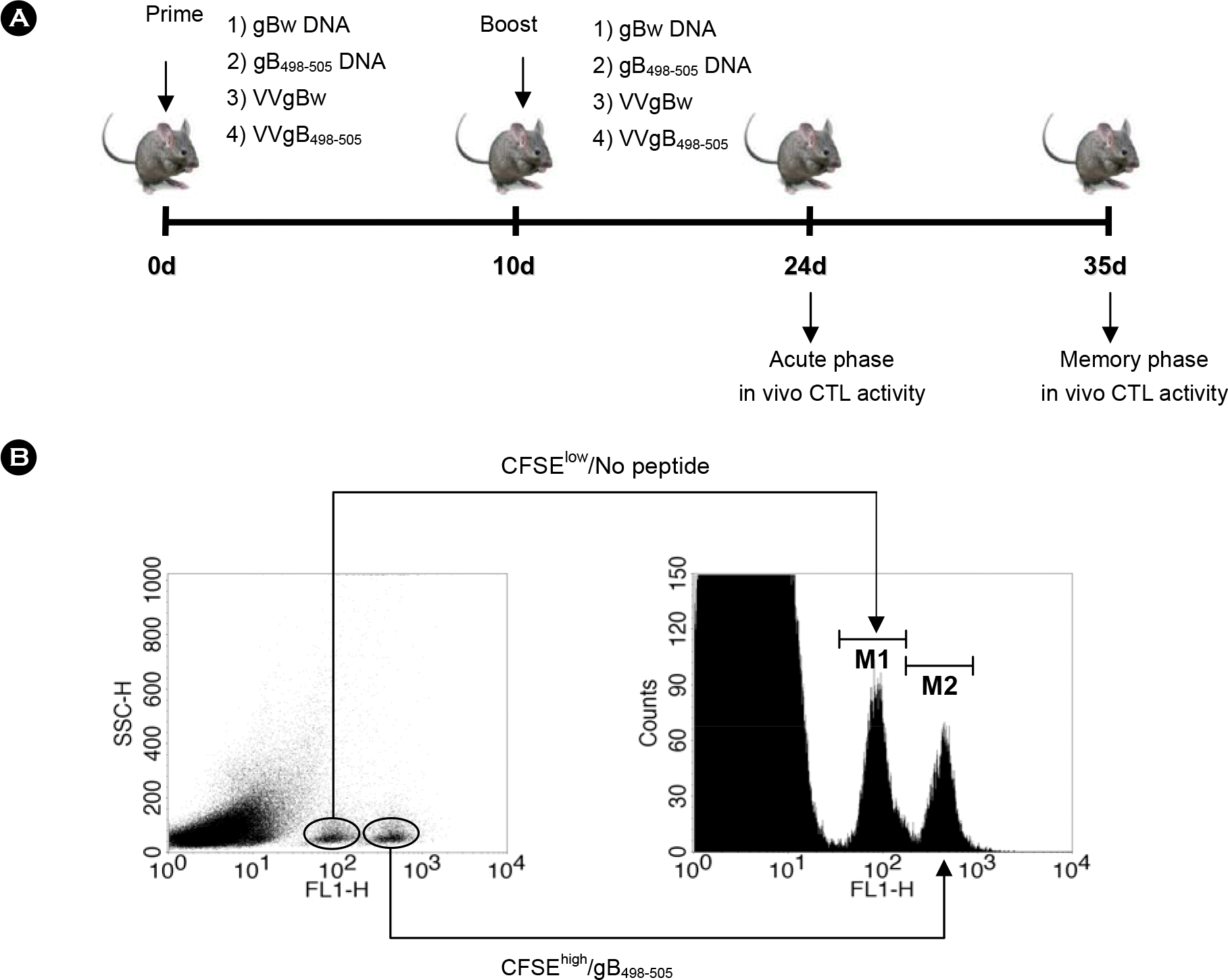 | Figure 1.(A) Diagram for prime-boost immunization. C57BL/6 (n=6∼7) were immunized i.m. with VVgB498–505, gB498–505 DNA, VVgBw, or gBw DNA, and boosted via the same route with the alternative vaccine type. Two (acute phase) and five (memory phase) weeks later, in vivo CTL killing activity was determined. (B) In vivo CTL killing assay for determining immunity mediated by epitope-specific CD8+ T cells. Splenocytes from naïve C57BL/6 mice (n=6∼7) were evenly split into two populations. One was plused with gB498–505 peptide (1 μg/ml) and then labeled with a high concentration (5.0 μM) of CFSE (CFSEhigh/gB498–505). The other was incubated without epitope peptide and labeled with with a low concentration (0.5 μM) of CFSE (CFSElow/No peptide). An equal number of cells from each population were mixed together and adoptively transferred into mice immunized with prime-boost protocols. Both CFSEhigh/gB498–505 and CFSElow/No peptide cells were analyzed with the flow cytometry 5 h after adoptive transfer. The histogram shows the reduced peak of CFSEhigh/gB498–505 due to cytolysis by epitope-specific CD8+ T cells. |
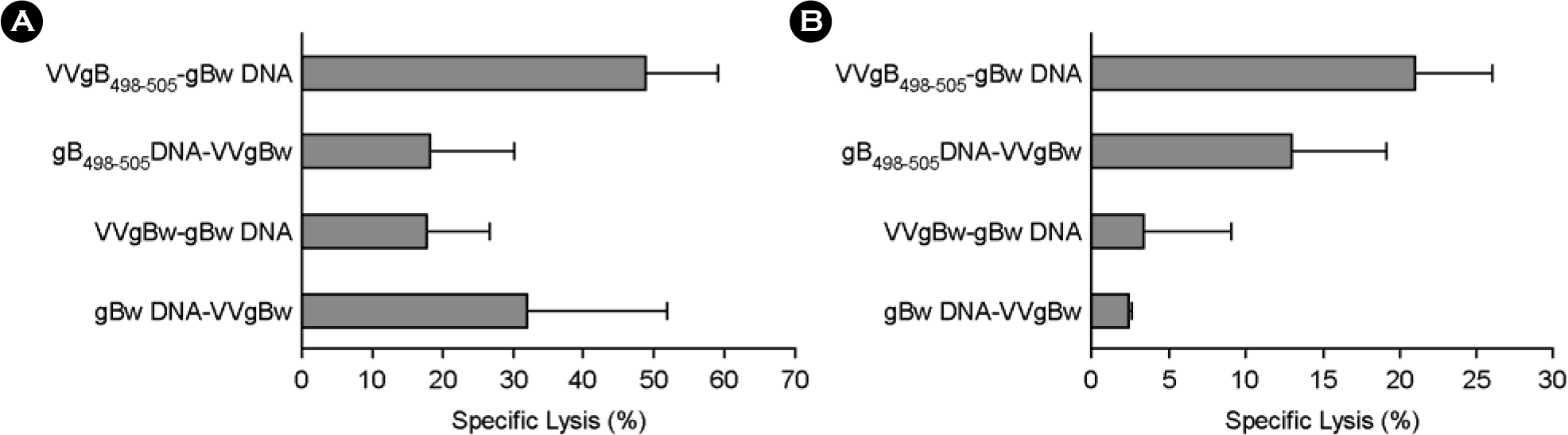 | Figure 2.In vivo CTL killing activity of C57BL/6 mice immunized with prime-boost protocols using recombinant vaccinia virus (VVgB498–505) and DNA vaccine (gB498–505) expressing gB498–505 epitope peptide at priming. C57BL/6 (n=6∼7) were immunized i.m. with VVgB498–505, gB498–505 DNA, VVgBw, or gBw DNA and boosted via the same route with either gBw DNA or VVgBw. Two (A; acute phase) and five (B; memory phase) weeks later, in vivo CTL killing activity was determined. The graphs show the average and standard deviation (SD) of three to four mice per experiment. |
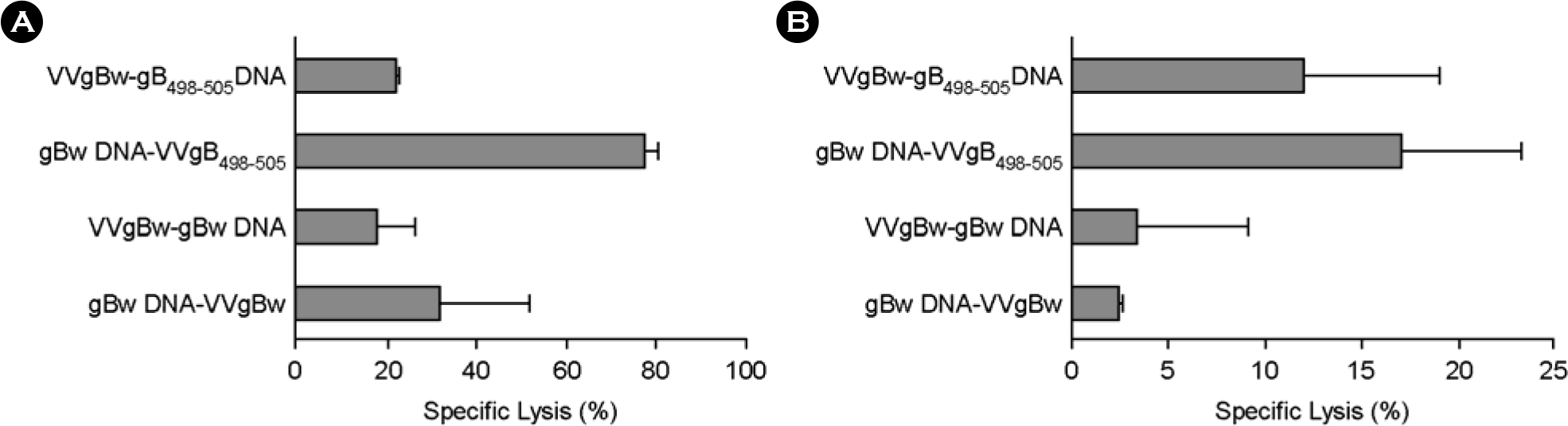 | Figure 3.In vivo CTL killing activity of C57BL/6 mice immunized with prime-boost protocols using recombinant vaccinia virus (VVgB498–505) and DNA vaccine (gB498–505) expressing gB498–505 epitope peptide at boosting. C57BL/6 (n=6∼7) were immunized i.m. with either VVgBw or gBw DNA, and boosted via the same route with VVgB498–505, gB498–505 DNA, VVgBw, or gBw DNA. Two (A; acute phase) and five (B; memory phase) weeks later, in vivo CTL killing activity was determined. The graphs show the average and standard deviation (SD) of three to four mice per experiment. |
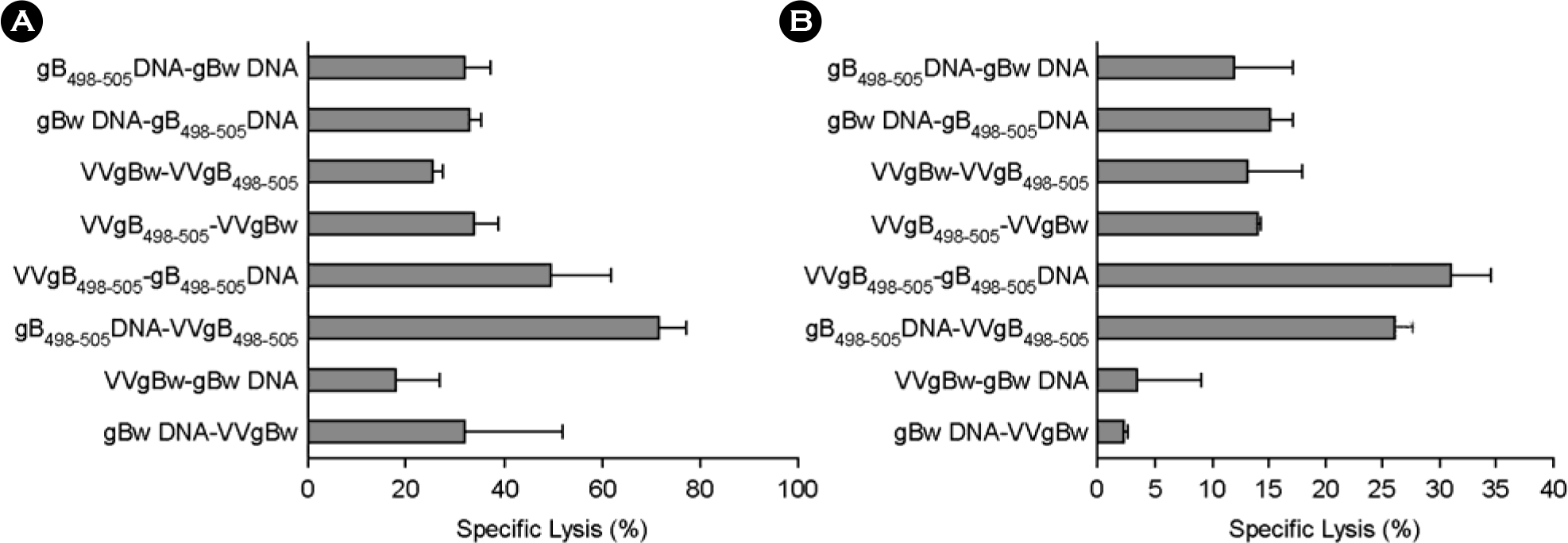 | Figure 4.In vivo CTL killing activity of C57BL/6 mice immunized with prime-boost protocols using recombinant vaccinia virus (VVgB498–505) and DNA vaccine (gB498–505) expressing gB498–505 epitope peptide at priming and boosting. C57BL/6 (n=6∼7) were immunized i.m. with VVgB498–505, gB498–505 DNA, VVgBw, or gBw DNA, and boosted via the same route with the alternative vaccine type. Two (A; acute phase) and five (B; memory phase) weeks later, in vivo CTL killing activity was determined. The graphs show the average and standard deviation (SD) of three to four mice per experiment. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download