Abstract
Recombinant DNA vaccines, based on plasmid vectors expressing an antigen under the control of a strong promotor, have several advantages over traditional vaccines. They have been shown to induce a full spectrum of immune responses for humoral and cellular systems and to secure the higher safety and the simplicity of administration. Thus, establishment of DNA vaccines against Newcastle disease virus (NDV) in poultry has been widely investigated using various virus strains and vector systems. In this study, the F and HN genes of NDV CBP-1 strains isolated from diseased pheasants and attenuated by serial passages in egg embryos were cloned using pSLIA vector and constructed two recombinants of pSLIA-tsF and pSLIA-tsHN. The recombinant plasmids were transfected into COS-7 cell and the expression of HN and F proteins were verified by immunofluorescence, SDS-PAGE and Western blot. The recombinant plasmids were injected intramuscularly and intradermally into C57B/6 mouse and a significant increment of HN and F antibodies was detected by ELISA. According to the results, it was implicative that the recombinant DNA could be utilized for development of recombinant DNA vaccine for NDV.
References
2). Babiuk LA, van Drunen Littel-van den Hurk S, Babiuk SL. Immunization of animals: from DNA to the dinner plate. Vet Immunol Immunopathol. 72:189–202. 1999.

3). Babiuk LA, Babiuk SL, Loehr BI, van Drunnen Littel-van den Hurk S. Nucleic acid vaccines: research tool or commercial reality. Vet Immunol Immunopathol. 76:1–23. 2000.

4). Bos JD. The skin as an organ of immunity. Clin Exp Immunol. 107:3–5. 1997.
5). Bruckner L, Stauber N, Brechtbuhl K, Hofmann MA. Detection of extraneous agents in vaccines using the polymerase chain reaction of Newcastle disease virus in poultry biologicals. Dev Biol Stand. 86:175–182. 1996.
6). Chang KS, Kim JY, Kim S, Kim TY, Song HJ, Jun MH. Expression of F protein gene of a thermostable isolate of Newcastle disease virus using Baculovirus expression system. Journal of Bacteriology and Virology. 31(2):163–174. 2001.
7). Chang KS, Kwak KH, Jand SI, Kim JY, Kim TY, Song YH, Song HJ, Jun MH. Molecular cloning and nucleotide sequence of the gene encoding hemagglutinin-neuraminidase (HN) of Newcastle disease virus isolated from a diseased pheasant in Korea. Korean J Vet Serv. 25:245–257. 2002.
8). Colman PM, Hoyne PA, Lawrence MC. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 67:2972–2980. 1993.

9). Dufour V. DNA vaccines: new applications for veterinary medicine. Vet Sci Tomorrow. 1:1–19. 2001.
10). Fang WH, Liang XY. Expression of the Newcastle disease virus fusion glycoprotein in vero cells using attenuated Salmonella typhimurium as transgenic carrier. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao. 34:488–493. 2002.
11). Fodor I, Horvath E, Fodor N, Nagy E, Rencendorsh A, Vakharia VN, Dube SK. Induction of protective immunity in chickens immunized with plasmid DNA encoding infectious bursal disease virus antigens. Acta Vet Hung. 47:481–492. 1999.
12). Homhuan A, Prakongpan S, Poomvises P, Maas RA, Crommelin DJA, Kersten GFA, Jiskoot W. Virosome and ISCOM vaccines against Newcastle disease; preparation, characterization and immunogenicity. Eur J Phamacol Sci. 22:459–468. 2004.

13). Kamiya N, Niitura M, Ono M, Kai C, Matsuura Y, Mikami T. Protective effect of individual glycoproteins of Newcastle disease virus expressed in insect cells; the fusion protein derived from an avirulent strain had lower protective efficacy. Virus Res. 32:373–379. 1994.
14). Karaca K, Sharma JM, Winslow BJ, Junker DE, Reddy S, Cochran M, McMillen J. Recombinant fowl pox viruses coexpressing chicken type I IFM and Newcastle disease virus HN and F genes; influence of IFN on protective efficacy and humoral responses of chickens following in ovo or post-hatch administration of recombinant viruses. Vaccine. 16(16):1496–1503. 1998.
15). Kodihalli S, Haynes JR, Robinson HL, Webster RG. Cross-protection among lethal H5N2 influenza viruses induced by DNA vaccine to the hemagglutinin. J Virol. 71:3391–3396. 1997.

16). Mayo MA. Virus Taxonomy-Houston 2002. Arch Virol. 147:5. 2002a.
18). Murakami Y, Kagino T, Niikura M, Mikami T, Ishii K, Matsuura Y. Characterization of Newcastle disease virus envelope glycoproteins expressed in insect cells. Virus Res. 33:123–137. 1994.
19). Nagy E, Huber P, Krell PJ, Derbyshire JB. Synthesis of Newcastle disease virus-like envelopes in insect cells infected with a recombinant baculovirus expressing the hemagglutininneuraminidase of NDV. J Gen Virol. 72:753–756. 1991.
20). Nishino Y, Niikura M, Suwa T, Onuma M, Gotoh B, Nagai Y, Mikami T. Analysis of the protective effect of the hemagglutinin-neuraminidase protein in Newcastle disease virus infection. J Gen Virol. 71:1187–1190. 1991.
21). Oshop GL, Elankumaran S, Heckert RA. DNA vaccination in the avian. Vet Immunol Immunopathol. 89:1–12. 2002.

22). Panda A, Elankumaran S, Krishnamurthy S, Huang Z, Samal SK. Loss of N-liked glycosylation from the hemagglutinin-neuraminidase protein alters virulence of Newcastle disease virus. J Virol. 78(10):4965–4975. 2004.
23). Reddy SK, Sharma JM, Ahmad J, Reddy DN, McMillen JK, Cook SM, Wild MA, Schwartz RD. Protective efficacy of a recombinant herpesvirus of turkeys as an in ovo vaccine against Newcastle and Marek's diseases in specific-pathogen-free chickens. Vaccine. 14:469–477. 1996.
24). Robinson HL. Nucleic acid vaccines: an overview. Vaccine. 15:785–787. 1997.
25). Sakaguchi M, Nakamura H, Sonoda K, Okamura H, Yokogawa K, Matsuo K, Hira K. Protection of chickens with or without maternal antibodies against both Marek's and Newcastle diseases by one-time vaccination with recombinant vaccine of Marek's disease virus type 1. Vaccine. 16(5):472–479. 1998.

26). Stauber N, Brechtbuhi K, Bruckner L, Hofmann MA. Detection of Newcastle disease virus in poultry vaccines using the polymerase chain reaction and direct sequencing of amplified cDNA. Vaccine. 13:360–364. 1995.

27). Stone-Hulslander J, Morrison TG. Detection of an interaction between the HN and F proteins in Newcastle disease vius-infected cell. J Virol. 71(9):6287–6295. 1997.
28). Suarez DL, Schultz-Cherry S. The effect of eukaryotic expression vectors and adjuvants on DNA vaccines in chickens using an avian influenza model. Avian Dis. 44:861–868. 2000.

29). Tan WS, Lau CH, Ng BK, Ibrahim AL, Yusoff K. Nucleotide sequence of the hemagglutinin-neuraminidase (HN) gene of a Malaysian heat resistant viscerotropic-velogenic Newcastle disease virus (NDV) strain AF2240. DNA Seq. 6(1):47–50. 1995.
30). Taylor J, Edbauer C, Rey-senelonge A, Bouquet JF. Norton E, Goebel S, Desmettre P, Paoletti E. Newcastle disease virus fusion protein expressed in a fowl pox virus recombinant confers protection in chickens. J Virol. 64:1441–1450. 1990.
31). Thacker EL, Fulton JE, Hunt HD. In vitro analysis of a primary, major histocompatibility complex (MHC)-restricted, cytotoxic T-lymphocyte response to avian leukosis virus (ALV), using target cells expressing MHC class I cDNA inserted into a recombinant ALV vector. J Virol. 69:6439–6444. 1995.
32). Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 259:1745–1749. 1993.

33). Wu YT, Peng DX, Liu XF, Liu WZ, Zhang RK. A recombinant fowl pox virus expressing the fusion protein of Newcastle disease virus strain F48E8 and its protective efficacy. Sheng Wu Gong Cheng Xue Bao. 16:591–594. 2000.
Figure 1.
Amplification patterns of NDV F gene (A) and HN gene (B) by the RT-PCR. Lane M: 1 kb DNA ladder marker, lane 1: CBP-1, lane 2: Hitchner B1, lane 3: LaSota-IB strain.
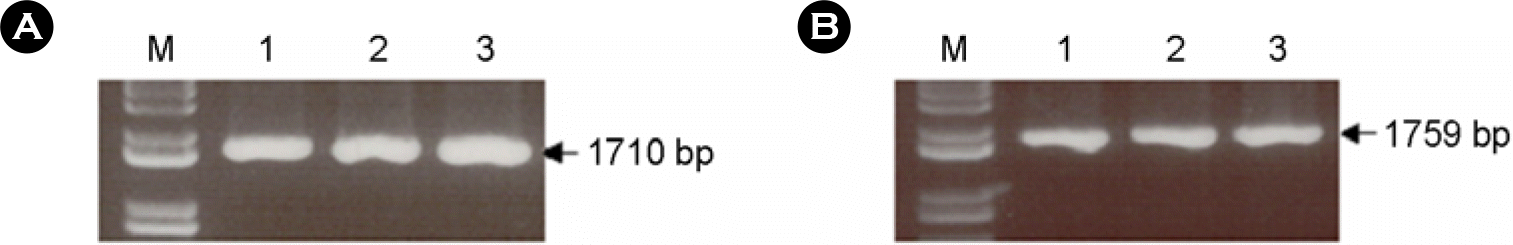
Figure 2.
Cleavage patterns of F gene (A) and HN gene (B) inserted into pSLIA by various restriction endonucleases. Lane M: 1 kb DNA ladder marker, lane 1: BamHI, lane 2: EcoRI, lane 3: PstI, lane 4: EcoRV.
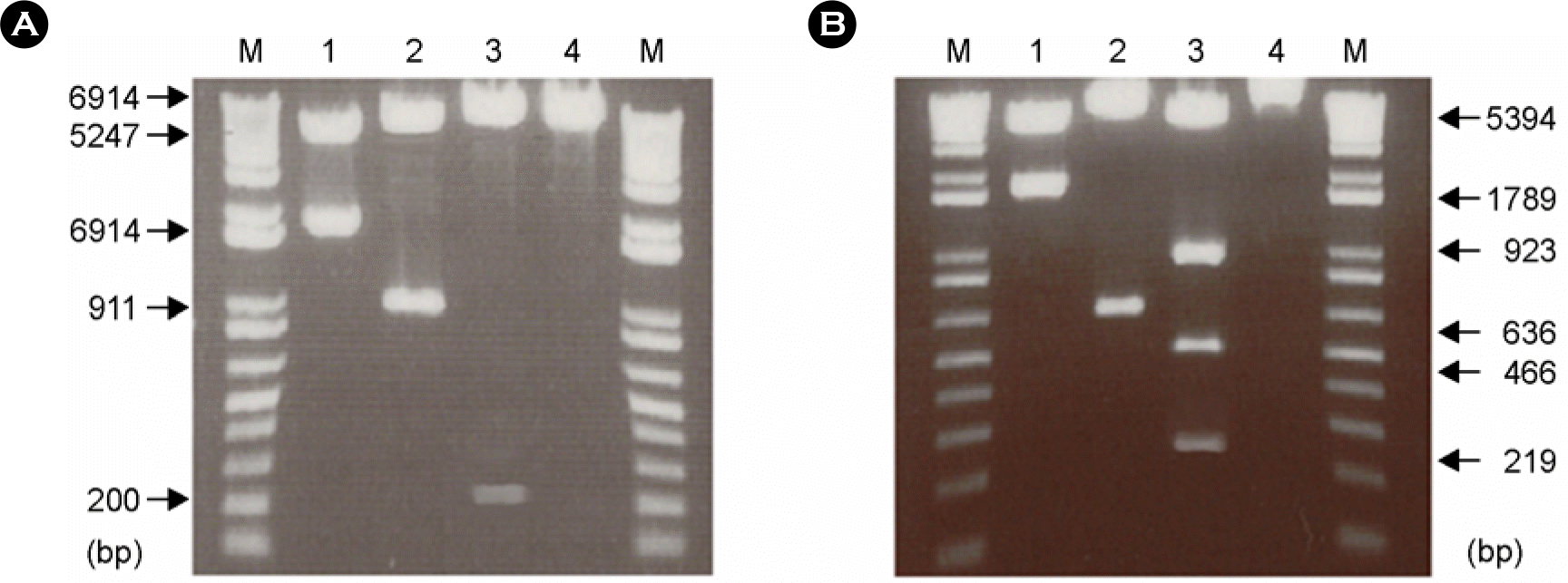
Figure 3.
Detection of F and HN proteins expressed by recombinant pSLIA-tsF and pSLIA-tsHN by immunofluorescent assay using monoclonal antibodies. A: Control cells, B: pSLIA-tsF-transfected COS-7 cells, C: pSLIA-tsHN-transfected COS-7 cells.
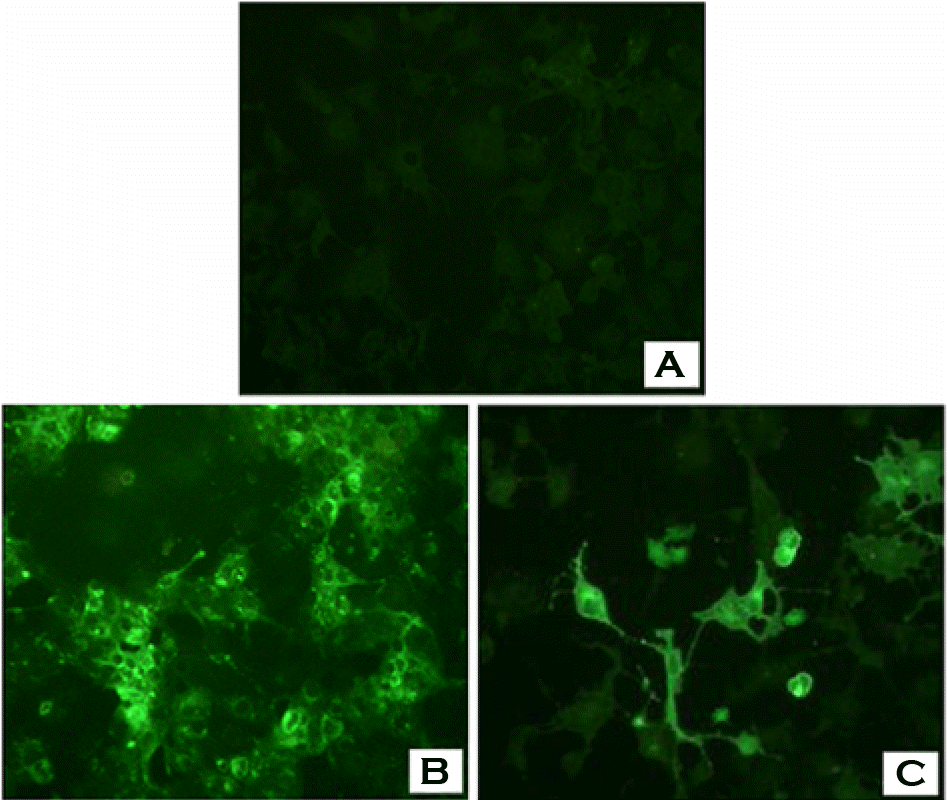
Figure 4.
SDS-PAGE patterns of F and HN proteins in COS-7 cell transfected with pSLIA-tsF and pSLIA-tsHN plasmids. Lane M: pre-stained protein marker, lane 1: pSLIA-tsF, lane 2: pSLIA, lane 3: Control, lane 4: pSLIA-tsHN, lane 5: pSLIA, lane 6: Control.
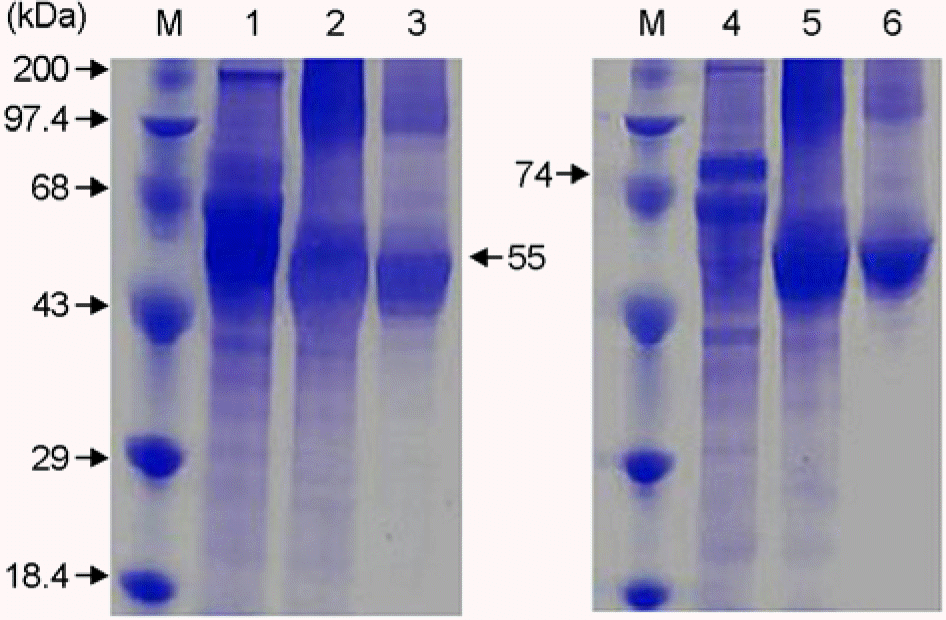
Figure 5.
Western blot analysis of F and HN proteins in COS-7 cell transfected with pSLIA-tsF and pSLIA-tsHN plasmids. Lane M: pre-stained protein marker, lane 1: pSLIA-tsF, lane 2: pSLIA, lane 3: Control, lane 4: pSLIA-tsHN, lane 5: pSLIA, lane 6: Control.
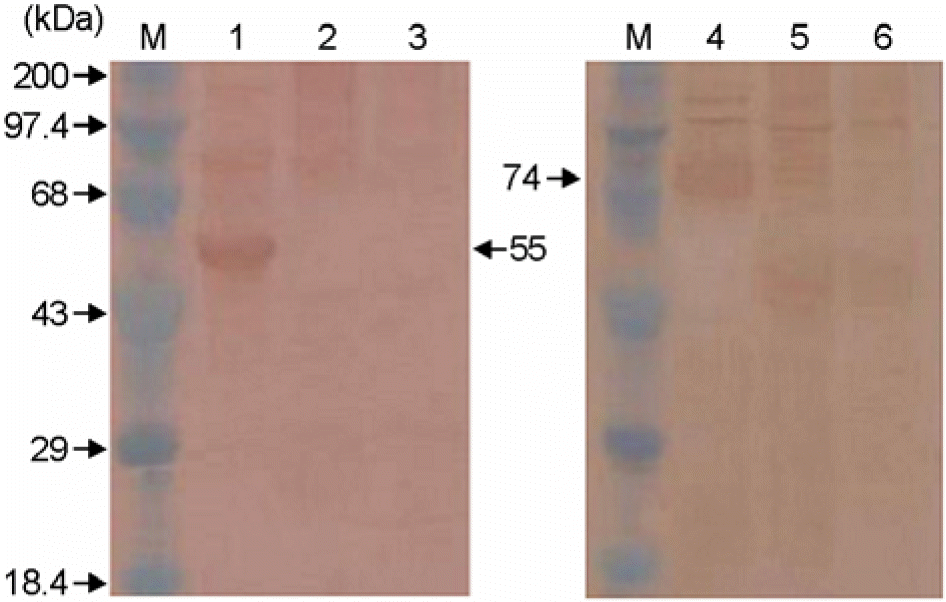
Figure 6.
Comparison of ELISA values in the mice injected by intramuscular (IM) and intradermal (ID) routes with the pSLIA-tsF and pSLIA-tsHN. ELISA values were measured at 6 weeks after immunization with the plasmids as shown in Table 1. I and II: injected intramuscularly, III and IV: injected intradermally, V (control): injected intramuscularly with pSLIA alone.
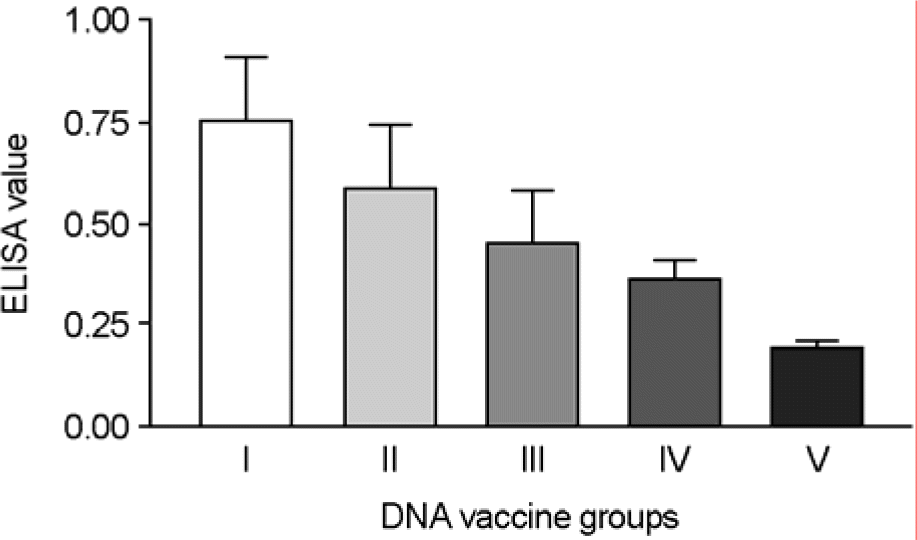
Table 1.
Immunization of the mice against recombinant DNA with F and HN genes
| Groups∗ | Immunogens | No. of mouse | Routes | Dose at weeks (μg) | |||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||||
| I | pSLIA-tsF | 5 | IM | 100 | 100 | 100 | Sacrificed |
| II | pSLIA-tsHN | 5 | IM | 100 | 100 | 100 | Sacrificed |
| III | pSLIA-tsF | 5 | ID | 100 | 100 | 100 | Sacrificed |
| IV | pSLIA-tsHN | 5 | ID | 100 | 100 | 100 | Sacrificed |
| V | Control | 5 | IM | 100 | 100 | 100 | Sacrificed |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download