Abstract
We previously demonstrated that the lentivirus lytic peptide 1 (LLP-1) corresponding to the carboxyl terminus of HIV-1 gp41 induced cell death in human neuronal cells. Present study was conducted to further elucidate the pathogenic mechanisms involved in HIV-1 gp41-induced neurodegeneration in AIDS patients with cognitive deficits. The effect of LLP-1 on activation of calpain-1, a calcium-activated cysteine protease, which has been implicated in neuronal degeneration and death, was monitored by the proteolysis of spectrin in rat organotypic hippocampal slice cultures. Protease specific spectrin breakdown products revealed that LLP-1 generated ∼150/145-kDa fragments characteristic of calpain-1 activation in hippocampus undergoing cell death as evidenced by LDH release. This spectrin cleavage pattern was further confirmed by in vitro calpain-1 proteolysis. Futhermore, calpectin and MDL28170, inhibitors of calpain activity, blocked calpain-1-mediated spectrin cleavage. Spectrin cleavage likely occurred in the absence of overt synaptic loss, as suggested by the preserved levels of synaptophysin. Among pharmacological agents tested, apocynin, NADPH oxidase inhibitor, ameliorated the LLP-1-induced spectrin. Given the role of spectrin essential for synapse stabilization, LLP-1-induced spectrin cleavage as occurs with the activation of calpain-1 may be an important effector in LLP-1-mediated cell injury in hippocampus, which is primarily linked to cognitive dysfunction.
Go to : 
References
1). Adamson DC, McArthur JC, Dawson TM, Dawson VL. Rate and severity of HIV-associated dementia (HAD): correlations with Gp41 and iNOS. Mol Med. 5:98–109. 1999.

2). Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 61:369–376. 2004.

3). Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 281:20315–20325. 2006.
4). Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 38:755–762. 1995.

6). Holopainen IE, Lauren HB. Neuronal activity regulates GABAA receptor subunit expression in organotypic hippocampal slice cultures. Neuroscience. 118:967–974. 2003.

7). Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 117:97–107. 2001.

8). Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 21:1–17. 2006.

9). Kalia V, Sarkar S, Gupta P, Montelaro RC. Rational site-directed mutations of the LLP-1 and LLP-2 lenti-virus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J Virol. 77:3634–3646. 2003.

10). Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 410:988–994. 2001.

11). Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 4:307–318. 2006.

12). Kelly BL, Vassar R, Ferreira A. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J Biol Chem. 280:31746–31753. 2005.
13). Kort JJ. Impairment of excitatory amino acid transport in astroglial cells infected with the human immunodeficiency virus type 1. AIDS Res Hum Retro. 14:1329–1339. 1998.

14). Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 1:101–110. 2004.

15). Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 12(Suppl 1):893–904. 2005.

16). Miller MA, Cloyd MW, Liebmann J, Rinaldo C, Islam Jr KR, Wang SZ, Mietzner TA, Montelaro RC. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology. 196:89–100. 1993a.

17). Miller MA, Mietzner TA, Cloyd MW, Robey WG, Montelaro RC. Identification of a calmodulin-binding and inhibitory peptide domain in the HIV-1 transmembrane glycoprotein. AIDS Res Hum Retro. 9:1057–1066. 1993b.

18). Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 4:435–452. 2005.

19). O'Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 26:981–990. 2006.
20). Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 60:377–385. 2001.

21). Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 15:918–928. 2005.

22). Ray SK, Hogan EL, Banik NL. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res Brain Res Rev. 42:169–185. 2003.

23). Rostasy K, Monti L, Yiannoutsos C, Kneissl M, Bell J, Kemper TL, Hedreen JC, Navia BA. Human immunodeficiency virus infection, inducible nitric oxide synthase expression, and microglial activation: pathogenetic relationship to the acquired immunodeficiency syndrome dementia complex. Annals Neurol. 46:207–216. 1999.

24). Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol (Berl). 107:97–110. 2004.
25). Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 23:11322–11331. 2003.

26). Sung JH, Shin SA, Park HK, Montelaro RC, Chong YH. Protective effect of glutathione in HIV-1 lytic peptide 1-induced cell death in human neuronal cells. J Neurovirol. 7:454–465. 2001.

27). Valle M, Price RW, Nilsson A, Heyes M, Verotta D. CSF quinolinic acid levels are determined by local HIV infection: cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain. 127:1047–1060. 2004.

28). Yamashima T. Ca2+-dependent proteases in ischemic neuronal death: a conserved “calpain-cathepsin cascade” from nematodes to primates. Cell Calcium. 36:285–293. 2004.
Go to : 
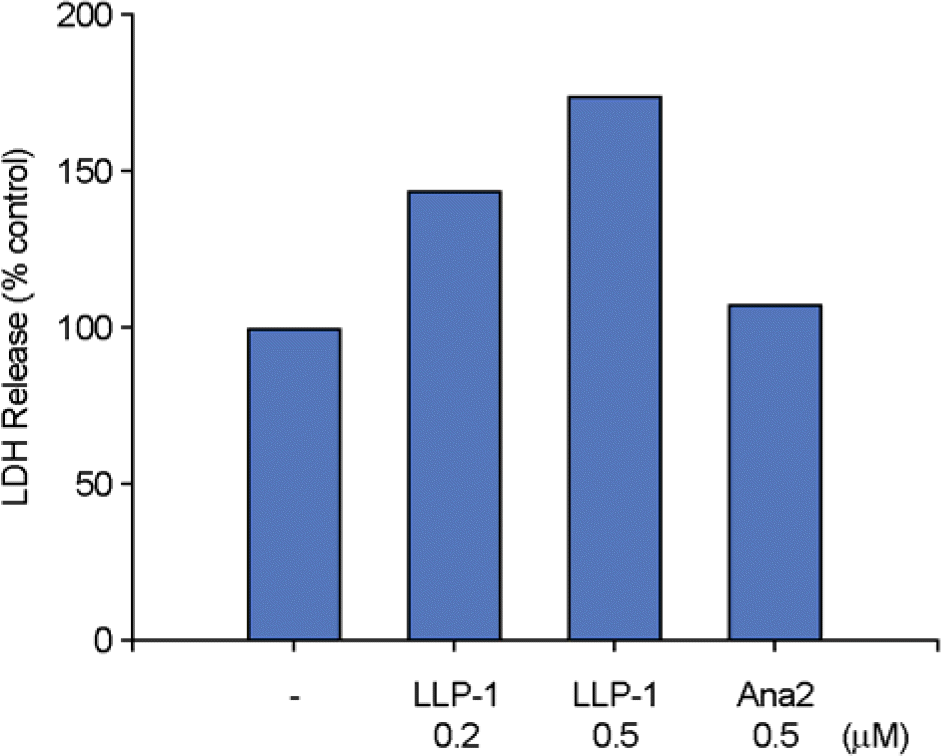 | Figure 1.LLP-1 increases LDH release in cultured media of organotypic hippocampal slice cultures. Dose response of LDH release by LLP-1 was examined by incubation with increasing concentrations of peptides as indicated for 30 h. LDH activity in cultured media from hippocampal slices was assayed after exposing rat hippocampal slice cultures to gp41 derived LLP-1 or LLP-1 analog 2 (Ana2). Values are expressed as a percentage of the vehicle treated controls, considering the values obtained in the hippocampal slices as 100%. |
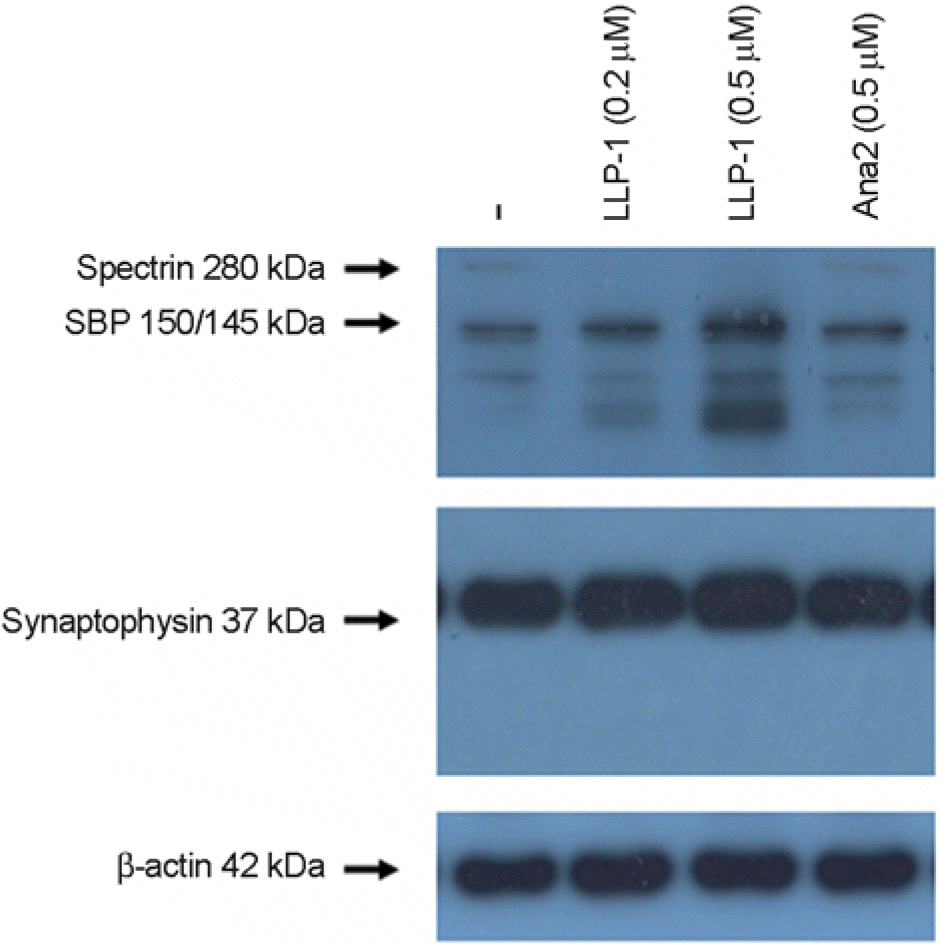 | Figure 2.LLP-1-induced cell death was accompanied by calpain-1 activation as analyzed by spectrin cleavage. Western blot analysis of spectrin and synaptophysin content in whole cell extracts prepared from hippocampal slices as described in Fig. 1 was performed as detailed in Materials and Methods section. LLP-1 treatment for 30 h clearly generated ∼150/145-kDa spectrin breakdown products (SBP) characteristic of calpain-1 activation. Representative blots from two to three experiments with similar results are shown. Uniformity of gel loading was confirmed with β-actin as the standard. |
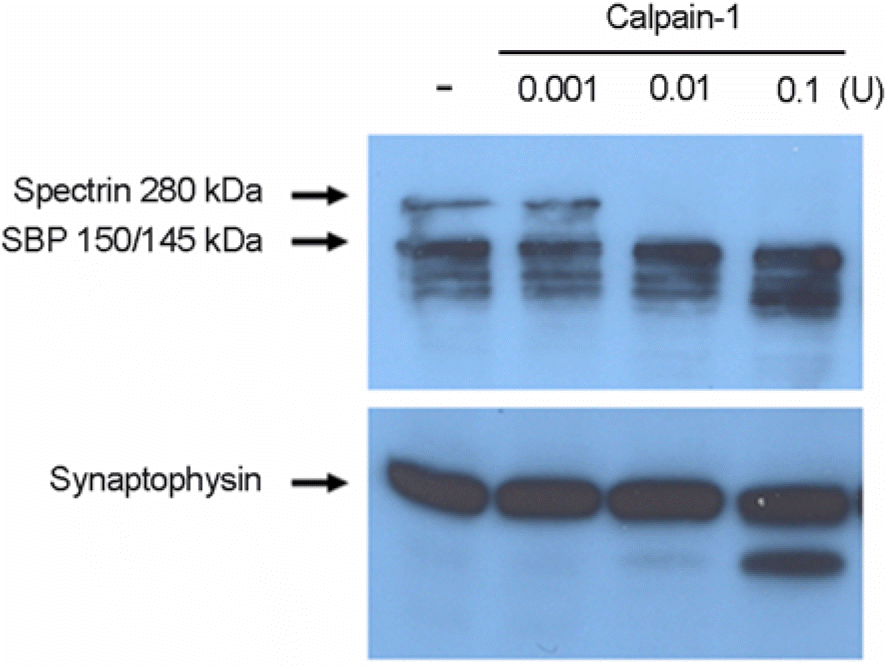 | Figure 3.
In vitro calpain-1 proteolysis confirms the spectrin cleavage pattern induced by LLP-1. Representative immunoblots of in vitro spectrin proteolysis by calpain-1. Spectrin or synaptophysin present in total lysate of hippocampal slices treated with vehicle only for 30 h are digested with increasing concentrations of calpain-1 for 1 h as indicated. Western blot analysis of spectrin and synaptophysin contents was performed as described in Fig. 2. Representative blots from two to three experiments with similar results are shown. |
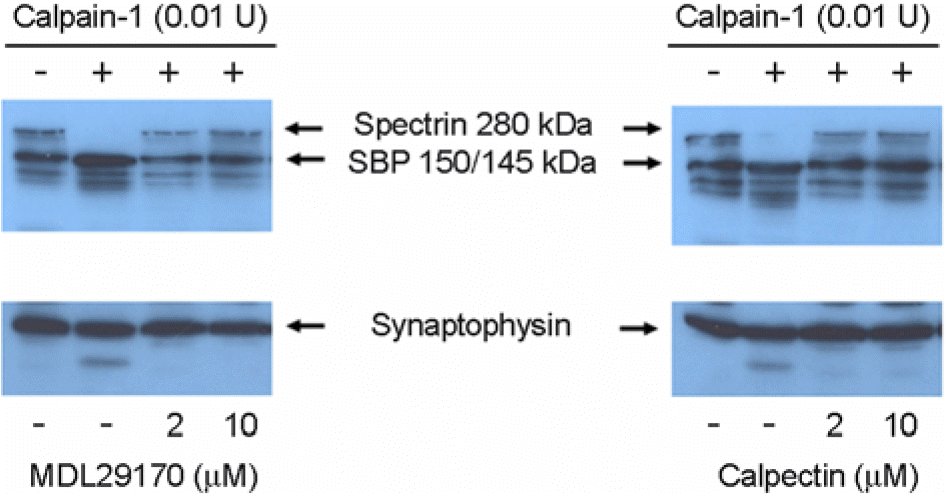 | Figure 4.Calpain-1 inhibitors blocked the spectrin cleavage pattern. Effects of specific calpain-1 inhibitors, calpectin and MDL-28170 on spectrin proteolysis in vitro by calpain-1 were analyzed. Whole cell extracts prepared from hippocampal slices treated with vehicle only were digested with calpain-1 in the presence of either calpectin or MDL28170 as indicated for 1 h. Western blot analysis of spectrin and synaptophysin contents was performed as described in Fig. 2. Representative blots from two to three experiments with similar results are shown. |
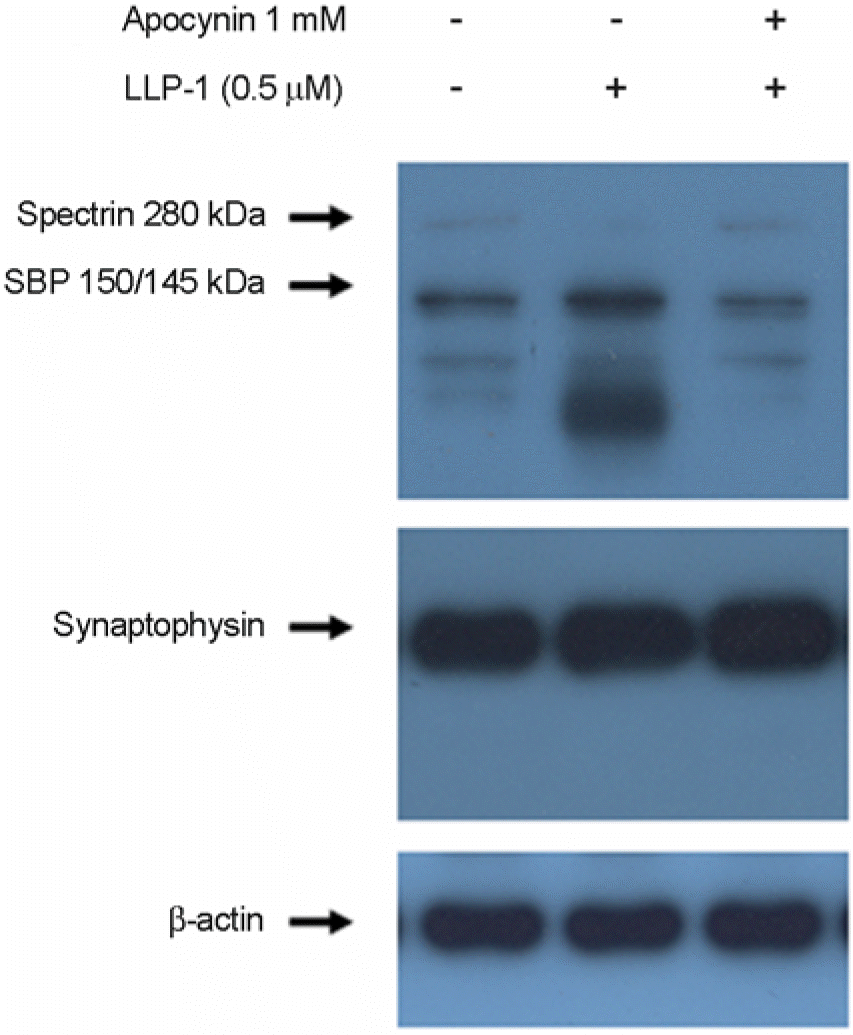 | Figure 5.Apocynin reduced the LLP-1-induced spectrin cleavage. Effects of apocynin, NADPH inhibitor, on spectrin proteolysis triggered by LLP-1 in hippocampal slices were analyzed by 1 h pretreatment with apocynin before exposure of hippocampal slices to LLP-1 for 30 h. Western blot analysis of spectrin content in whole cell extracts prepared from hippocampal slices was conducted as described in Fig. 1 and Fig. 2. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download