Abstract
The purification of immunodominant native protein antigens from the culture filtrates of Mycobacterium tuberculosis is needed for the development of new vaccines and immunodiagnostic reagents against tuberculosis. In the present study, we conducted large scale purification of well-known secreted antigens, Ag85 complex, 38-kDa, and MTB12, from the culture filtrate proteins (CFPs) prepared from M. tuberculosis H37Rv grown as a surface pellicle on synthetic Sauton medium. The protein and antigen concentrations of culture filtrates were sufficiently increased after 6 week of culture. The MTB12 antigen was detected as early as 1 week of culture, and Ag85 complex and 38-kDa antigen were detected after 2 and 3 week of culture, respectively, by immunodiffusion with specific antiserum against 100-fold concentrated culture filtrates. For large-scale purification, the six-week-culture filtrates of M. tuberculosis H37Rv diluted 2.5-fold with 20 mM Tris-HCl, pH 8.3 were subjected to anion-exchange chromatography. The CFPs were eluted with 100 mM NaCl-20 mM Tris-HCl, pH 8.3 and concentrated by ultrafiltration. The concentrated CFPs were fractionated with ammonium sulfate, and followed by hydrophobic interaction chromatography and anion-exchange chromatography (FPLC). Eventually, 10 mg of Ag85 complex, 0.56 mg of 38-kDa, and 1.81 mg of MTB12 antigens were purified from 1 liter of the six-week-culture filtrates of M. tuberculosis H37Rv which contained 307.81 mg of protein of culture filtrate.
Go to : 
References
1). Andersen P, Askgaard D, Ljungqvist L, Bentzon MW, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 59:1558–1563. 1991.
2). Andersen P. The T cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology. 191:537–547. 1994.
3). Andersen AB, Hansen EB. Structure and mapping of anti-genic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 57:2481–2418. 1989.
4). Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 191:520–525. 1994.
5). Barnes PF, Mehra V, Rivoire B, Fong SJ, Brennan PJ, Voegtline MS, Minden P, Houghten RA, Bloom BR, Modlin RL. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J Immunol. 148:1835–1840. 1992.
6). Boesen H, Jensen BN, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 63:1491–1497. 1995.
7). Cardoso FL, Antas PR, Milagres AS, Geluk A, Franken KL, Oliveira EB, Teixeira HC, Nogueira SA, Sarno EN, Klatser P, Ottenhoff TH, Sampaio EP. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infect Immun. 70:6707–6714. 2002.
8). Chang Z, Choudhary A, Lathigra R, Quiocho FA. The immunodominant 38-kDa lipoprotein antigen of Mycobacterium tuberculosis is a phosphate-binding protein. J Biol Chem. 269:1956–1958. 1994.
9). Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 271:698–702. 1994.
10). Daniel TM, Janicki BW. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 42:84–113. 1978.

11). Davidow A, Kanaujia GV, Shi L, Kaviar J, Guo X, Sung N, Kaplan G, Menzies D, Gennaro ML. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect Immun. 73:6846–6851. 2005.
12). Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 282:677–686. 1999.
16). Fonseca DP, Benaissa-Trouw B, van Engelen M, Kraaijeveld CA, Snippe H, Verheul AF. Induction of cell-mediated immunity against Mycobacterium tuberculosis using DNA vaccines encoding cytotoxic and helper T-cell epitopes of the 38-kilodalton protein. Infect Immun. 69:4839–4845. 2001.
17). Giulian GG, Moss RL, Greaser M. Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem. 142:421–436. 1984.

18). Haga S, Yamaguchi R, Nagai S, Matsuo K, Yamazaki A, Nakamura RM. Delayed-type hypersensitivity to a recombinant mycobacterial antigen, MPB64, in guinea pigs sensitized to Mycobacterium tuberculosis or Mycobacterium bovis BCG. J Leukoc Biol. 57:221–225. 1995.
19). Harth G, Lee BY, Wang J, Clemens DL, Horwitz MA. Novel insights into the genetics, biochemistry, and immuno-cytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 64:3038–3047. 1996.
20). Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 92:1530–1534. 1995.
21). Kaufmann SH. Is the development of a new tuberculosis vaccine possible? Nat Med. 6:955–960. 2000.

22). Lee BY, Horwitz MA. T-cell epitope mapping of the three most abundant extracellular proteins of Mycobacterium tuberculosis in outbred guinea pigs. Infect Immun. 67:2665–2670. 1999.
23). Lee JS, Son JW, Jung SB, Kwon YM, Yang CS, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Ex Vivo Responses for Interferon-gamma and Proinflammatory Cytokine Secretion to Low-Molecular-Weight Antigen MTB12 of Mycobacterium tuberculosis during Human Tuberculosis. Scand J Immunol. 64:145–154. 2006.
24). Lim JH, Park JK, Jo EK, Song CH, Min D, Song YJ, Kim HJ. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect Immun. 67:6187–6190. 1999.
25). Mehra V, Bloom BR, Bajardi AC, Grisso CL, Sieling PA, Alland D, Convit J, Fan XD, Hunter SW, Brennan PJ, et al. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 175:275–284. 1992.
26). Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 72:6148–6150. 2004.

27). Pehler K, Brasky KM, Butler TM, Attanasio R. Mycobacterium tuberculosis-secreted protein antigens: immunogenicity in baboons. J Clin Immunol. 20:306–316. 2000.
28). Rosenkrands I, Weldingh K, Ravn P, Brandt L, Hojrup P, Rasmussen PB, Coates AR, Singh M, Mascagni P, Andersen P. Differential T-cell recognition of native and recombinant Mycobacterium tuberculosis GroES. Infect Immun. 67:5552–5558. 1999.
29). Samanich KM, Keen MA, Vissa VD, Harder JD, Spencer JS, Belisle JT, Zolla-Pazner S, Laal S. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 7:662–668. 2000.
30). Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 157:679–691. 1998.

31). Silveira H, Ordway D, Dockrell H, Jackson M, Ventura F. Cell-mediated immune responses to mycobacterial antigens in patients with pulmonary tuberculosis and HIV infection. Clin Exp Immunol. 110:26–34. 1997.

32). Skjot RL, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun. 68:214–220. 2000.
33). Stenger S, Modlin RL. T cell mediated immunity to Mycobacterium tuberculosis. Curr Opin Microbiol. 2:89–93. 1999.
34). Triccas JA, Roche PW, Winter N, Feng CG, Butlin CR, Britton WJ. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 64:5171–5177. 1996.
35). Webb JR, Vedvick TS, Alderson MR, Guderian JA, Jen SS, Ovendale PJ, Johnson SM, Reed SG, Skeiky YA. Molecular cloning, expression, and immunogenicity of MTB-12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis. Infect Immun. 66:4208–4214. 1998.
Go to : 
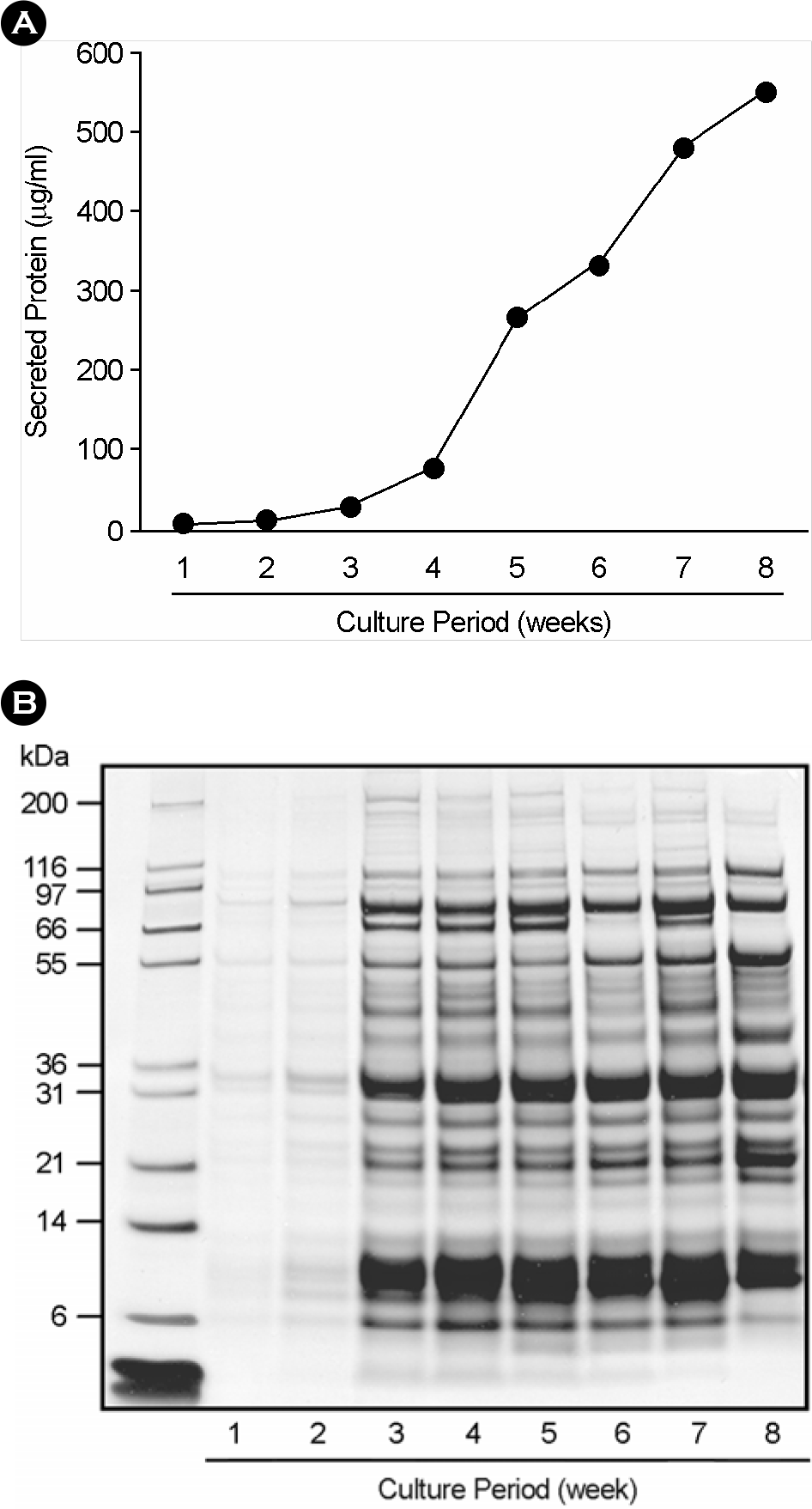 | Figure 1.Protein concentration and SDS-PAGE analysis of culture filtrates from M. tuberculosis H37Rv. (A) The protein concentration of culture filtrates during different culture period (1∼8 week). The culture filtrates of M. tuberculosis H37Rv were isolated from growing tubercle bacilli in Sauton's synthetic medium. And the protein concentrations were measured by bicinchoninic acid (BCA) protein assay. (B) SDS-PAGE analysis of culture filtrates during various culture periods. The culture filtrates for SDS-PAGE analysis were concentrated 100-fold by ultrafiltration. The 70 μg of protein was loaded to gradient Bis-Tris gel (4∼12%), except culture filtrates for 1∼2 weeks (10 μg of protein), separated by SDS-PAGE, and Coomassie blue stained. Lane 1∼8; week 1∼8, respectively. |
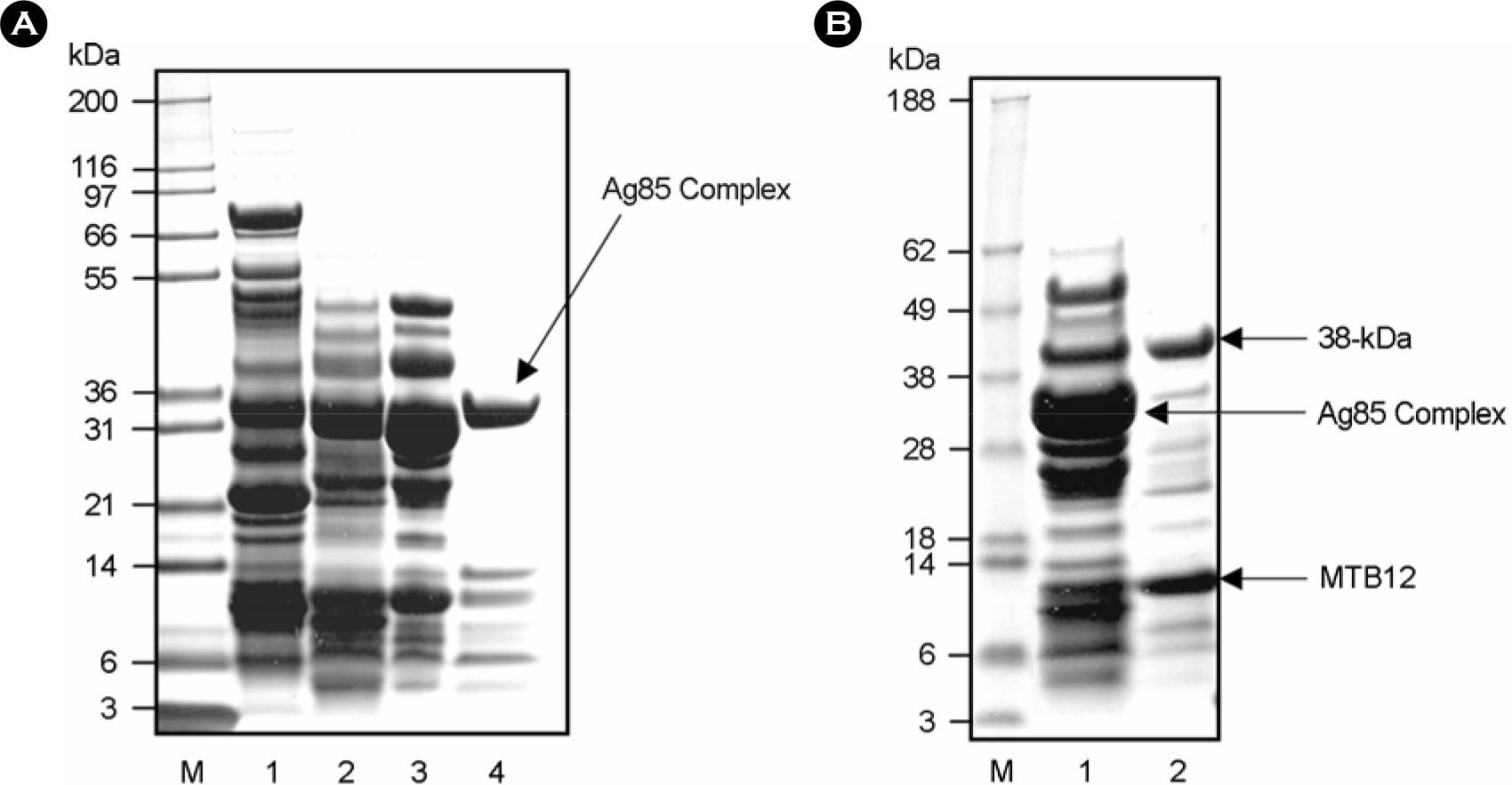 | Figure 2.(A) SDS-PAGE analysis of M. tuberculosis culture filtrate protein concentration by anion-exchange chromatography. The unheated culture filtrate protein of M. tuberculosis H37Rv was concentrated by anion-exchange chromatography. The culture filtrate protein of M. tuberculosis was diluted with 2.5 volume of 20 mM Tris-HCl, pH 8.3 and loaded to a column (2.5 by 20 cm) of Macro-prep High Q support (Bio-Rad). The CFPs were eluted in 20 mM Tris-HCl, pH 8.3 with 100 mM NaCl, concentrated by ultrafiltration (1st elution; lane 1). All pass-through fractions produced in the procedure were pooled, and then reapplied to Macro-prep High Q column, eluted with 100 mM NaCl again and concentrated by ultrafiltration (2nd elution; lane 2). This process was repeated twice more (3rd and 4th elution; lane 3, 4) (B) SDS-PAGE analysis after ammonium sulfate fractionation of the culture filtrate proteins (CFPs). Lane 1, 0∼40% ammonium sulfate fraction; lane 2, 40∼80% ammonium sulfate fraction. |
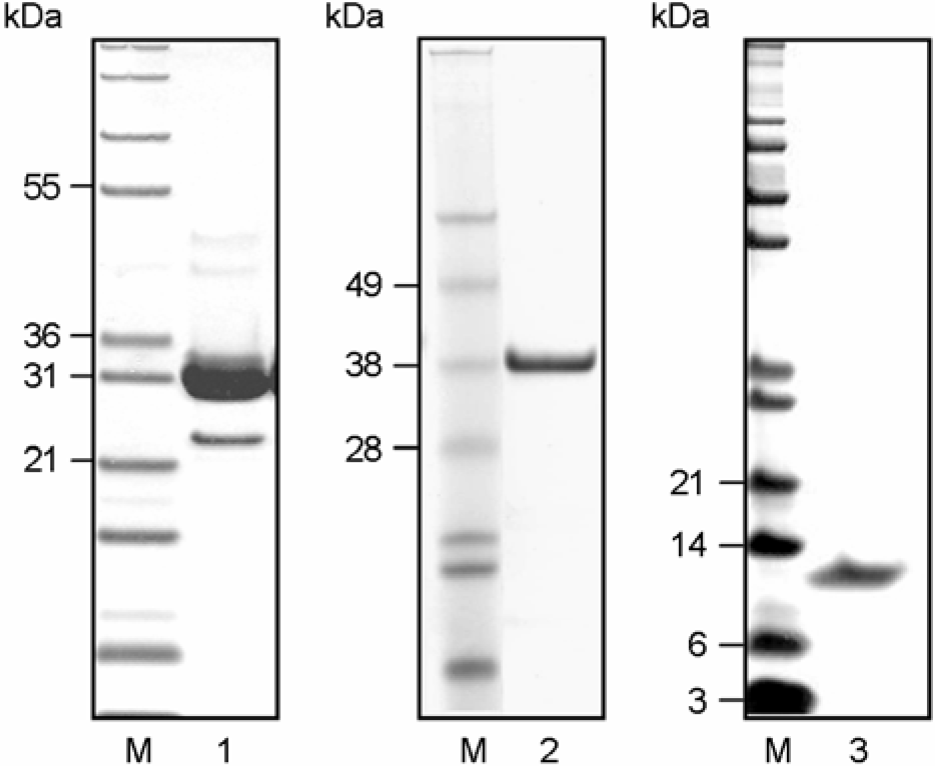 | Figure 3.SDS-PAGE analysis of finally purified Ag85 complex, 38-kDa, and MTB12 antigen of M. tuberculosis culture filtrate. The Ag85 complex, 38-kDa, and MTB12 were purified from unheated culture filtrate of M. tuberculosis H37Rv by 1st anion-exchange chromatography (Macro-prep high Q supported column (Bio-Rad), ultrafiltration, ammonium sulfate fractionation, hydrophobic interaction chromatography and 2nd anion-exchange chromatography (UNO-Q6 column (Bio-Rad) (FPLC). Lane 1, purified Ag85 complex; Lane 2, purified 38-kDa Ag; lane 3, purified MTB12 Ag. |
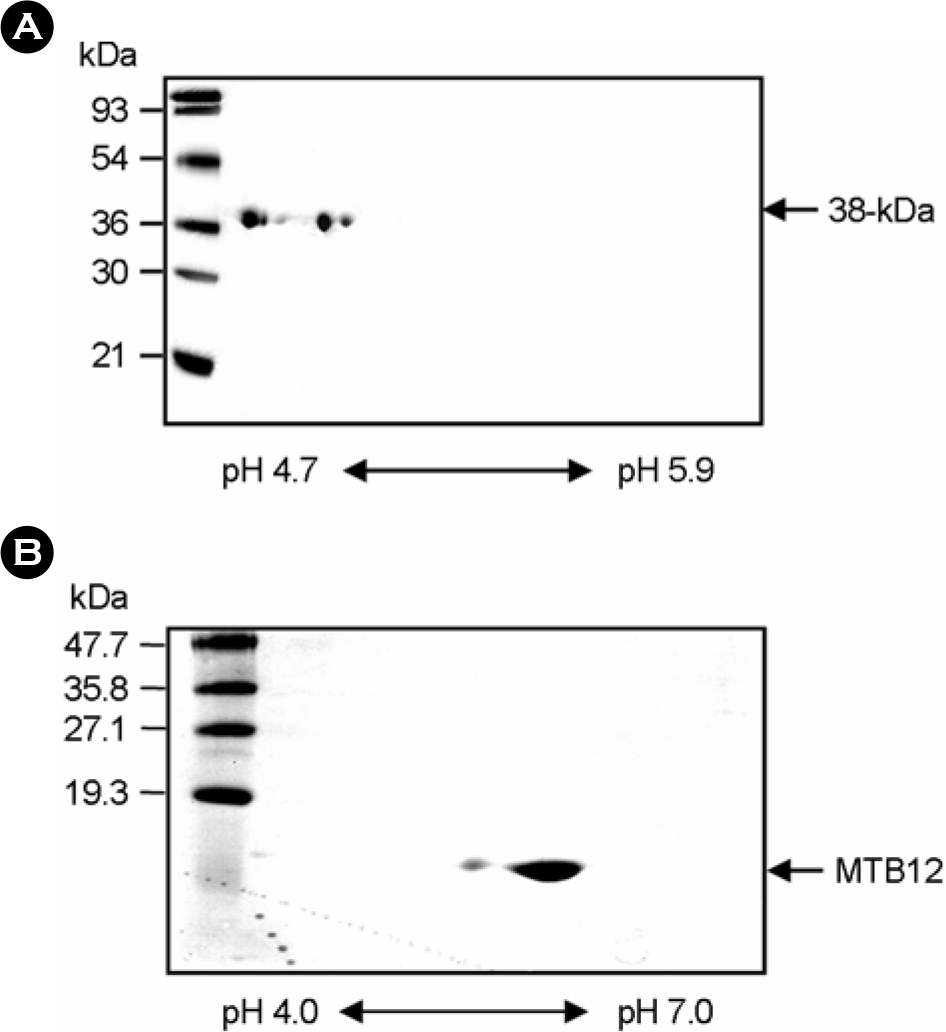 | Figure 4.Two-dimensional gel electrophoresis analysis of the purified 38-kDa and MTB12 antigens purified from M. tuberculosis culture filtrate. The purified 38-kDa and MTB12 antigens were resolved by isoelectrofocusing (38-kDa Ag, pH 4.7∼5.9; MTB12, pH 4.0∼7.0) followed by SDS-PAGE (38-kDa Ag, 12% acrylamide gel; MTB12 Ag, 15% acrylamide gel). The gels were stained with Coomassie blue. |
Table 1.
The protein and antigen concentration of culture filtrates during the different culture period of M. tuberculosis H37Rv
| Duration of culture (weeks) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Protein concentration of culture filtrates (μg/ml) | 2.6 | 5.4 | 25.5 | 74.5 | 264.0 | 331.0 | 479.0 | 549.0 | |
| Titer of specific antigena | Ag85 complex | 0 | 8 | 16 | 64 | 128 | 128 | 128 | 256 |
| 38-kDa | 0 | 0 | 1 | 2 | 8 | 16 | 16 | 64 | |
| MTB12 | 2 | 4 | 32 | 128 | 256 | 512 | 512 | 512 | |
Table 2.
The purification procedures of Ag85 complex, 38-kDa and MTB12 antigens from the culture filtrate of Mycobacterium tuberculosis
| Purification procedure | Ag85 complex | 38-kDa | MTB12 |
|---|---|---|---|
| Anion exchange chromatographya (Macro-prep high Q) | 100 mM NaCl | 100 mM NaCl | 100 mM NaCl |
| Ammonium sulfate fractionation | 0∼40% | 40∼80% | 40∼80% |
| Hydrophobic interaction chromatographyb | 0.81∼0.45 M | 1.35∼1.17 M | 1.8∼1.62 M |
| Anion exchange chromatographyc (UNO-Q) | 0∼20 mM NaCl | 0∼20 mM NaCl | 0∼10 mM NaCl |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download