Abstract
There are well known infectious diarrheal disease including viral disease such as HuCVs (Human caliciviruses), rotaviruses, enteric adenoviruses and astroviruses. We performed surveillance of infectious diarrheal disease in this study for providing the information for regional prevalence of infectious diarrheal disease and establish basement of diagnostic scheme for these infectious diarrheal disease. For the study, 3,400 stool specimens collected from 10 hospitals in Gwangju from April 2000 to March 2002 were used in investigation for the detection of infectious diarrheal disease. For group A rotaviruses, enteric adenoviruses and astrovirus, we carried out antigen capturing ELISA and RT-PCR with specific primers reacting RNA dependent RNA polymerase gene of HuCVs is used for the detection of RNA of HuCVs. As a results, we detected viral antigen or genome from 537 out of 3,400 specimens (15.8%). 443 out of 537 (82.5%) were confirmed as rotaviruses antigen positively, and 14 (2.6%) and 3 (0.8%) samples were antigen positive to enteric adenoviruses and astroviruses, respectively. We detected HuCV genome from 73 (13.6%) samples by specific amplification. We found that predominantly causative virus is rotavirus in Gwangju but HuCVs take major portion of viral agents causing diarrhea considering the age and seasonal distribution of specimens. Prevalence of adenoviruses and astroviruses are very low compared with worldwide situation. While the infection of rotavirus is limited to young infant under 2 years old, infection of HuCV has wide age distribution. These results suggest that existence of various strains of HuCVs and low rate of cross-protection among distinct antigenic group make it difficult to form immunity in older age. This epidemiological information relating to various diarrheic viruses is first reported in Gwangju, therefore it could provide present prevalence of viral agents causing gastroenteritis and backgrounds to establishment of protection viral diarrhea and development.
References
1). 김경희. Small Round-Structured Virus (SRSV)의 국내에서 의 중요도 및 국내 분리주 (Seoul-SRSV) 유전자의 염기 서열에 관한 연구. J K o re a n Soc Vi ro logy. 25:23–30. 1995.
2). 장미윤, 양재명, 김경희. Expression and Antigenicity of Replicase 단백의 발현 및 항원성 평가. J Korean Soc Vir ology. 27:151–160. 1997.
3). 지영미, 김기순, 천두성, 박정구, 강영화, 정윤석, 고운영, 신영학, 윤재득. 원주지역 설사환자에서 분리한 Small Round Structured Viruses (SRSV) 염기서열분석. J Korean Soc Virology. 29:247–259. 1999.
4). 지영미, 김기순, 천두성, 안정배, 강영화, 정윤석, 이선 화, 김운호, 윤재득. 바이러스성 설사질환의 역학조사 및 원인체 분석을 통한 예방대책 수립에 관한 연구. 국립보건원보. 37:96–112. 2000.
5). Allard A, Girones R, Juto P, Wadell G. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 28:2659–2667. 1997.

7). Barnes GL. Etiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol. 36:133–138. 1998.
8). Belliot G, Laveran H, Monroe SS. Detection and genetic differentiation of human astrovirus: phylogenetic grouping varies by coding region. Arch Virol. 142:1323–1334. 1997.
9). Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Detection of a new virus by electron microscopy of fecal extracts from children with acute gastroenteritis. Lancet. 1:149–151. 1974.
10). Bon F, Fascia P, Dauvergne M, Tenenbaum D, Planson H, Petion AM, Pothier P, Kohli E. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus Type 40 and 41 infections among children with gastroenteritis in Dijon, France. J Clin Microbiol. 37:3055–3058. 1999.
11). Brandt CD, Kim HW, Yolken RH. Comparative epidemiology of two rotavirus serotype and other viral agents associated with pediatric gastroenteritis. AM J Epidemiol. 110:243–254. 1979.
13). Caroline CS, Eduardo MV, Maria CMV, Fabiano MS, Tatiane RBC, Carlos MN, Rosa EL. Prevalence of enteric adenoviruses among children with diarrhea in four Brazilian cities. J Clin Virol. 23:171–177. 2002.
14). Centers for Disease Controll and Prevention. Intussusception among recipients of rotavirus vaccine in United States, 1998–1999. 48:577–581. 1999.
15). Cruz JR, Bartlett AV, Herrmann JE. Astrovirus associated diarrhea among Guatemalan ambulatory rural children. J Clin Microbiol. 30:1140–1144. 1992.
16). Dedman D, Laurichesse H, Caul EO, Wall PG. Surveillance of small round structured virus (SRSV) infection in England and wales, 1990–1995. Epidemiol Infect. 121:139–149. 1998.
17). Gentsch JR, Glass RI, Woods P. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 30:1365–1373. 1992.

18). Giordano M, Ferreyra LJ, Isa MB, Martinez LC, Yudowsky SI, Nates SV. The epidemiology of acute viral gastroenteritis in hospitalized children in Cordoba city, Argentina: An insight of disease burden. Rev Inst Med trop S Paulo. 43:193–197. 2001.

19). Glass RI, Bresee J, Jiang B, Gentsch J, Ando T, Frankhauser R, Noel J, Parashar U, Rosen B, Monroe SS. Gastroenteritis Viruses; an overview. Novartis Found Symp. 238:5–19. 2001.

20). Goggero A, O'Ryan M, Noel JS. Prevalence of astrovirus infection among Chilean children with acute gastroenteritis. J Clin Microbiol. 36:3691–3693. 1998.

21). Green J, Vinjé J, Gallimore CI, Koopmans M, Hale A, Brown DW. Capsid protein diversity among Norwalk-like viruses. Virus Genes. 20:227–236. 2000.
22). Guerrant RL, Hughes JM, Lima NL, Crane J. Diarrhea in developed and developing countries: magnitude, special settings and etiologies. Rev Infect Dis. 12:41–50. 1990.

23). Herrmann JE, Nowak NA, Perron-Henry DM. Diagnosis of astrovirus gastroenteritis by antigen detection with monoclonal antibodies. J Infect Dis. 161:226–229. 1990.

24). Hoehne M, Schreier E. Detection of Norovirus genogroup I and II by multiplex real-time RT-PCR using a 3′-minor groove binder-DNA probe. Infect Dis. 10:66–69. 2006.
25). Jarecki-Khan Km Tzipori SR, Unicomb LE. Enteric adeno-virus infection among infants with diarrhea in rural Bangladesh. J Clin Microbiol. 31:484–489. 1993.

26). Kapikian AZ, Wyatt RG, Dolin R. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 10:1075–1081. 1972.

27). Konno T, Suzuki H, Katsushima N. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis. 147:125–128. 1983.

28). Koopmans M, Vinje M, Leenen I, Vander PW, van Duynhoven Y. Molecular epidemiology of human enteric calici-viruses in the Netherlands. J Infect Dis. 181:262–269. 2000.

29). Lew JF, Petric M, Kapikian AZ, Jiang X, Estes MK, Green KY. Identification of minireovirus as a Norwalk-like viruses in pediatric patients with gastroenteritis. J Virol. 68:3391–3396. 1994.
30). Lewis HM, Parry JV, Davies HA. A year's exprience of the rotavirus syndrome and its association with respiratory illness. Arch Dis Child. 54:339–346. 1979.
31). Liu C, Grillner L, Jonsson K, Linde A, Shen K, Lindell AT, Wirgart BZ, Johansen K. Identification of viral agents associated with diarrhea in young children during a winter season in Beijing, China. J Clin Virol. 35:69–72. 2006.

32). Matsui SM, Greenberg HB. Astroviruses. In. Fields BN, Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin HA, Roizman B, Straus SE, editors. Virology. 4th ed.Lippincott williams & Wilkins;2001.
33). Moe CL, Gentch J, Ando T, Grohmann G, Monroe SS, Jiang X, Wang J, Estes MK, Seto Y, Hunphrey C, Stine S, Glass RI. Application of PCR to detect Norwalk-like virus in fecal speciments from outbreaks of gastroenteritis. J Clin Microbiol. 32:642–648. 1994.
34). Pan TG, Nguyen TA, Kuroiwa T, Kaneshi K, Ueda Y, Nakaya S, Nishimura S, Nishimura T, Yamamoto A, Okitsu S, Ushijima H. Viral diarrhea in Japanese children: results from a one-year epidemiologic study. Clin Lab. 51:183–191. 2005.
35). Pang XL, Preiksaitis JK, Lee B. Multiplex real time RT-PCR for the detection and quantitation of norovirus genogroups I and II in patients with acute gastroenteritis. J Clin Virol. 33(2):168–71. 2005.

37). Parashar UD, Gibson CJ, Breasea JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 12:304–306. 2006.

38). Parker TD, Kitamoto N, Tanaka T, Hutson AM, Estes MK. Identification of Genogroup I and Genogroup II broadly reactive epitopeson the norovirus capsid. J Virol. 79(12):7402–7409. 2005.
39). Saito H, Saito S, Kamada K, Harata S, Sato H, Morita M, Miyajima Y. Application of RT-PCR designed from the sequence of the Local SRSV strain to the screenining in viral gastroenteritis outbreaks. Microbiol Immunol. 42:439–446. 1998.
40). Uhnoo I, Wadell G, Svensson L, Johansson ME. Importance of enteric adenovirus 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 20:365–372. 1984.
41). Vesikari T, Maki M, Sarkkinen HK. Rotavirus, adenovirus, and non-viral enteropathogens in diarrhea. Arch Dis Child. 56:264–270. 1981.
Figure 1.
The result of gel electrophoresis for gene amplification of human caliciviruses using clinical specimenfrom diarrheal patients in Gwangju (A: primer panel based on the sequences from Norwalk and Snow mountain viruses, B: primer panel II based on the sequences from Yuri and Toronto viruses).
A; lane M: 1 kb DNA plus ladder (invitrogen) lane 1–7: clinical specimen
B; lane M: 1 kb DNA plus ladder (invitrogen) lane 1–2: clinical specimen
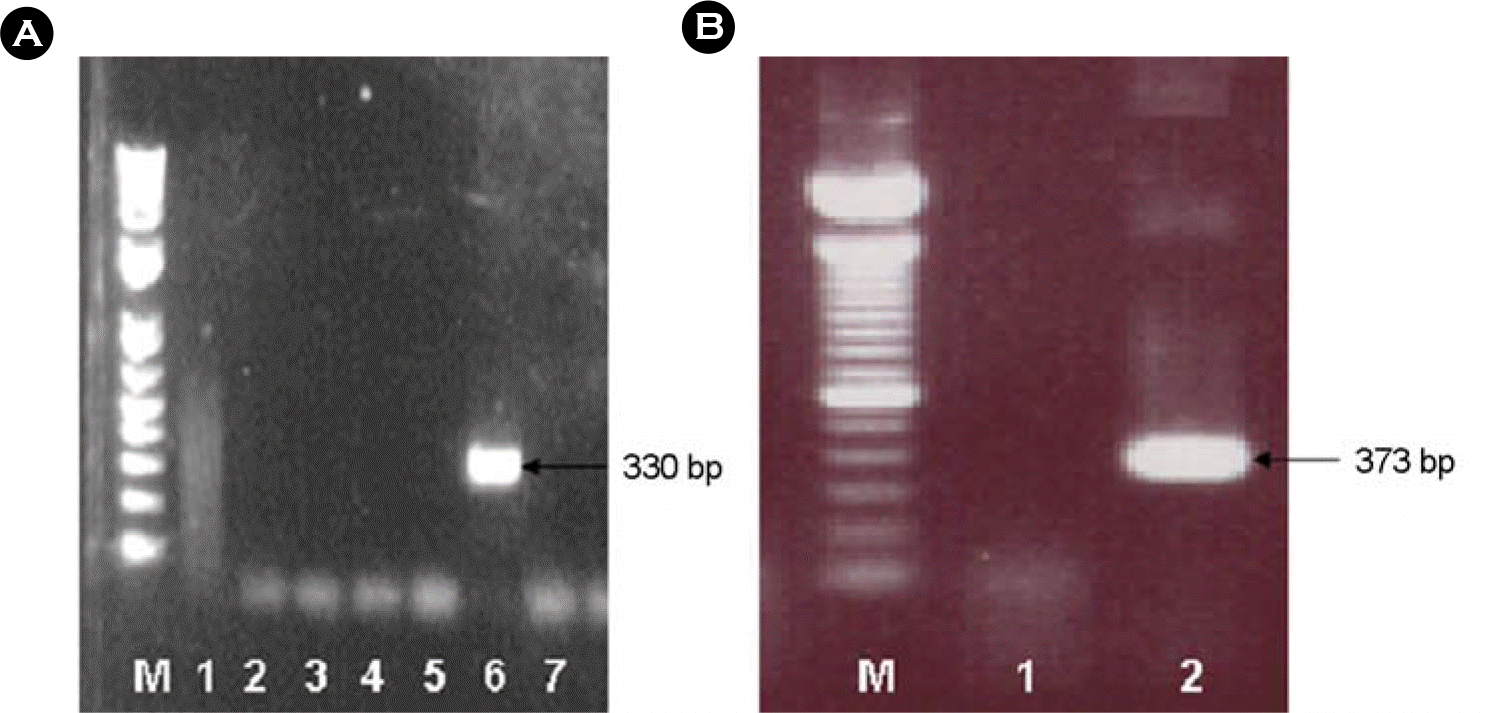
Figure 2.
The prevalence of diarrheic viruses from patinets with acute gastroenteritis in Gwangju during 2 years (2000∼2002).
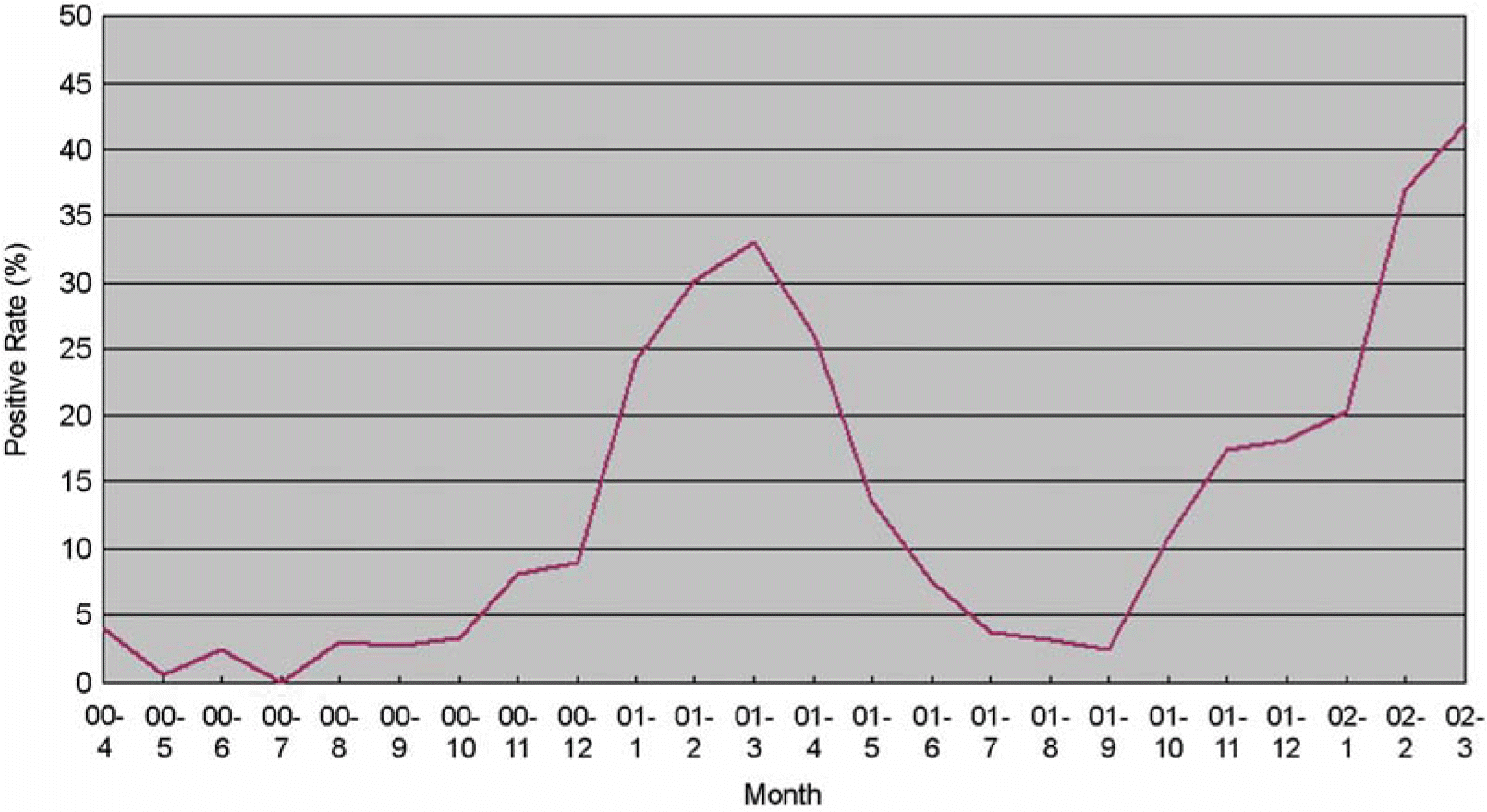
Table 1.
Oligonucleotide primers used to detect the HuCV
Table 2.
Distribution of virus detected by month (2000. 4∼2002. 3)
Table 3.
Number of viruses detected by age group (2004.∼2002. 3)
Table 4.
Number of virus detected by sex (2000. 4∼2002. 3)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download