Abstract
Xenotransplantation, as a potential solution to the shortage of human organs, is associated with a number of concerns including immunologic rejection and xenogenic infection. While the pigs are considered the most suitable organ source for xenotransplantation, there is a potential public health risk due to zoonosis. Among the known porcine zoonotic microbes, Porcine Endogenous Retrovirus (PERV) is the most considerable virus. PERV belongs to the Gammaretrovirus and has been divided into three groups (A, B, and C). To characterize the gag of PERVs, we isolated the genomic DNAs from three pig breeds (Birkshire, Duroc, and Yorkshire) and two types of SPF miniature pigs. About 1.5 kb fragments covering full length of gag were amplified and cloned into T-vector. A total of 38 clones were obtained and sequenced. Nucleotide sequences were analyzed and phylogenetic trees were constructed from the nucleotide and deduced amino acids. PERV-A, -B and -C were present in the proportion of 47, 19 and 34%, respectively. Regardless of origin or subgroups, gag clones showed highly homology in nucleotide and deduced amino acid sequences. Deduced amino acids sequence alignments showed typical conserve sequences, Cys-His box and processing sites. Among analyzed clones, about 28% of isolates had the correct open reading frame. To test the functional expression of Gag protein, gag was subcloned into expression vector and confirmed its expression in HeLa cell. This research provides the fundamental information about molecular characteristics of gag gene and functional Gag protein related xenotropic PERVs.
Go to : 
References
1). Akiyoshi DE, Denaro M, Zhu H, Greenstein JL, Banerjee P, Fishman JA. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol. 72:4503–4507. 1998.

2). Alin K, Goff SP. Amino acid substitutions in the CA protein of Moloney murine leukemia virus that block early events in infection. Virology. 222:339–351. 1996.

3). Blusch JH, Patience C, Martin U. Pig endogenous retro-viruses and xenotransplantation. Xenotransplantation. 9:242–251. 2002.

4). Breese SS Jr. Virus-like particles occurring in cultures of stable pig kidney cell lines. Brief Report Arch Gesamte Virusforsch. 30:401–404. 1970.

6). Cairns TM, Craven RC. Viral DNA synthesis defects in assembly-competent Rous sarcoma virus CA mutants. J Virol. 75:242–250. 2001.

7). Cannon PM, Matthews S, Clark N, Byles ED, Iourin O, Hockley DJ, Kingsman SM, Kingsman AJ. Structure-function studies of the human immunodeficiency virus type 1 matrix protein, p17. J Virol. 71:3474–3483. 1997.

8). Choi G, Park S, Choi B, Hong S, Lee J, Hunter E, Rhee SS. Identification of a cytoplasmic targeting/retention signal in a retroviral Gag polyprotein. J Virol. 73:5431–5437. 1999.

9). Craven RC, Leure-du Pree AE, Weldon RA Jr, Wills JW. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J Virol. 69:4213–4227. 1995.

10). Crawford S, Goff SP. Mutations in gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 49:909–917. 1984.

11). D'Souza V, Summers MF. How retroviruses select their genomes. Nat Rev Microbiol. 3:643–655. 2005.
12). Hanger JJ, Bromham LD, McKee JJ, O'Brien TM, Robinson WF. The nucleotide sequence of koala (Phascolarctos cinereus) retrovirus: a novel type C endogenous virus related to Gibbon ape leukemia virus. J Virol. 74:4264–4272. 2000.
13). Heneine W, Tibell A, Switzer WM, Sandstrom P, Rosales GV, Mathews A, Korsgren O, Chapman E, Folks TM, Groth CG. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 352:695–699. 1998.

14). Hall BG. Comparison of the accuracies of several phylogenetic methods using protein and DNA sequences. Mol Biol Evol. 22:792–802. 2005.

15). Jorgensen EC, Pedersen FS, Jorgensen P. Matrix protein of Akv murine leukemia virus: genetic mapping of regions essential for particle formation. J Virol. 66:4479–4487. 1992.

16). Blusch Jürgen H., Sigrid Seelmeir, Klaus von der Helm. Molecular and Enzymatic haracterization of the Porcine Endogenous Retrovirus Protease. J Virol. 76:7913–7917. 2002.
17). Young Kelly R., Ross Ted M.Elicitation of Immunity to HIV Type 1 Gag Is Determined by Gag Structure. AIDS RESEARCH AND HUMAN RETROVIRUSES. 22:99–108. 2006.

18). Lee EG, Alidina A, May C, Linial ML. Importance of basic residues in binding of rous sarcoma virus nucleocapsid to the RNA packaging signal. J Virol. 77:2010–2020. 2003.

19). Lee SK, Nagashima K, Hu WS. Cooperative effect of gag proteins p12 and capsid during early events of murine leukemia virus replication. J Virol. 79:4159–4169. 2005.

20). Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 389:681–682. 1997.

21). Magre S, Takeuchi Y, Bartosch B. Xenotransplantation and pig endogenous retroviruses. Rev Med Virol. 13:311–329. 2003.

22). Martina Y, Marcucci KT, Cherqui S, Szabo A, Drysdale T, Srinivisan U, Wilson CA, Patience C, Salomon DR. Mice transgenic for a human porcine endogenous retrovirus receptor are susceptible to productive viral infection. J Virol. 80:3135–3146. 2006.

23). Meric C, Goff SP. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Viro. 63:1558–1568. 1989.
24). Patience C, Patton GS, Takeuchi Y, Weiss RA, McClure MO, Rydberg L, Breimer ME. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet. 352:699–701. 1998.

25). Schwartzberg P, Colicelli J, Gordon ML, Goff SP. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 49:918–924. 1984.

26). Todaro GJ, Benveniste RE, Lieber MM, Sherr CJ. Characterization of a type C virus released from the porcine cell line PK(15). Virology. 58:65–74. 1974.

27). Tristem M, Kabat P, Lieberman L, Linde S, Karpas A, Hill F. Characterization of a novel murine leukemia virus-related subgroup within mammals. J Virol. 70:8241–8246. 1996.

28). Van de Peer Y, De Wachter R. Construction of evolutionary distance trees with TREECON for Windows: accounting for variation in nucleotide substitution rate among sites. Comput Appl Biosci. 13:227–230. 1997.

Go to : 
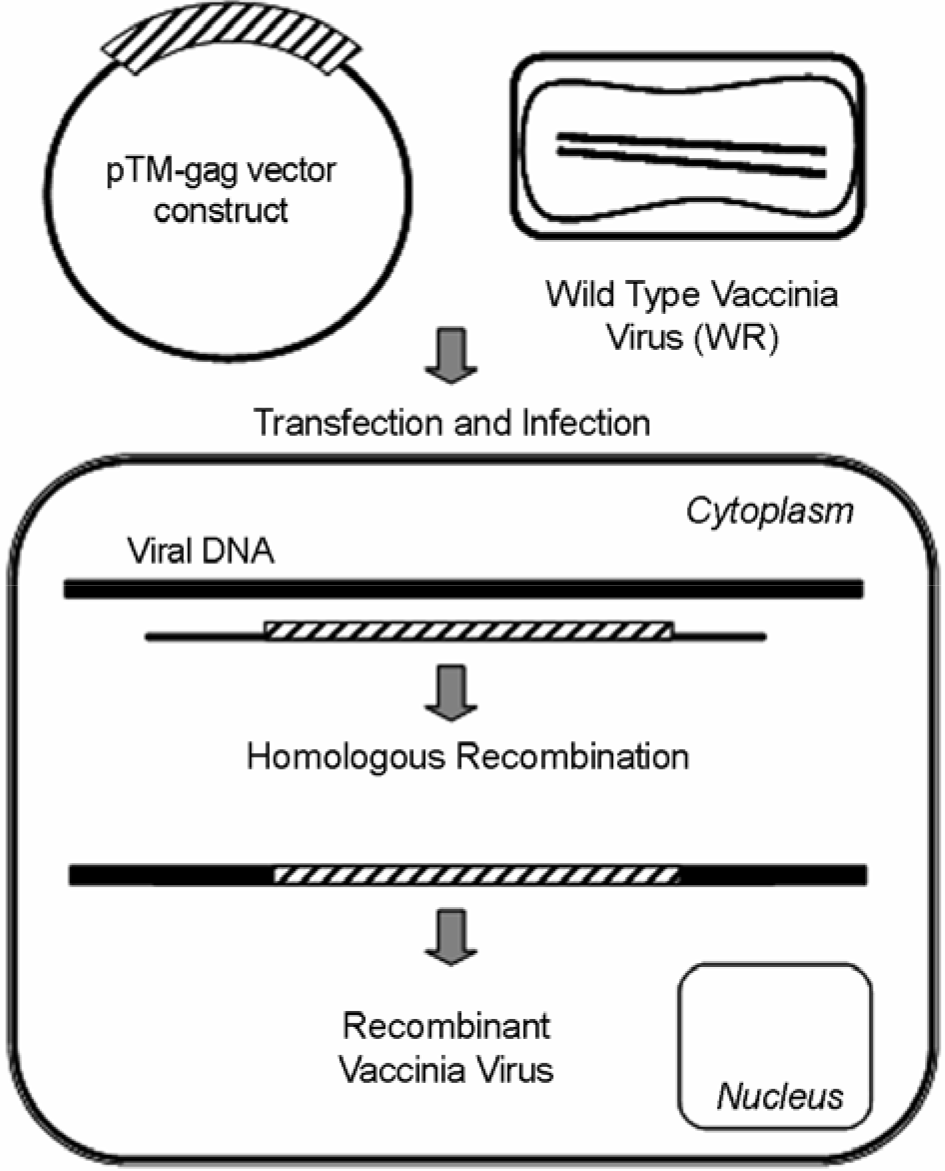 | Figure 1.Construction of vaccinia virus recombinant. HeLa cells transfeted with a PERV gag plasmid are infeceted with wild type vaccinia virus (WR). Homologous recombinantion occurs between flanking sequences beside gag gene and virus genomic DNA. In a consequence of PERV gag gene insertion into viral DNA, recombinant virus was produced. |
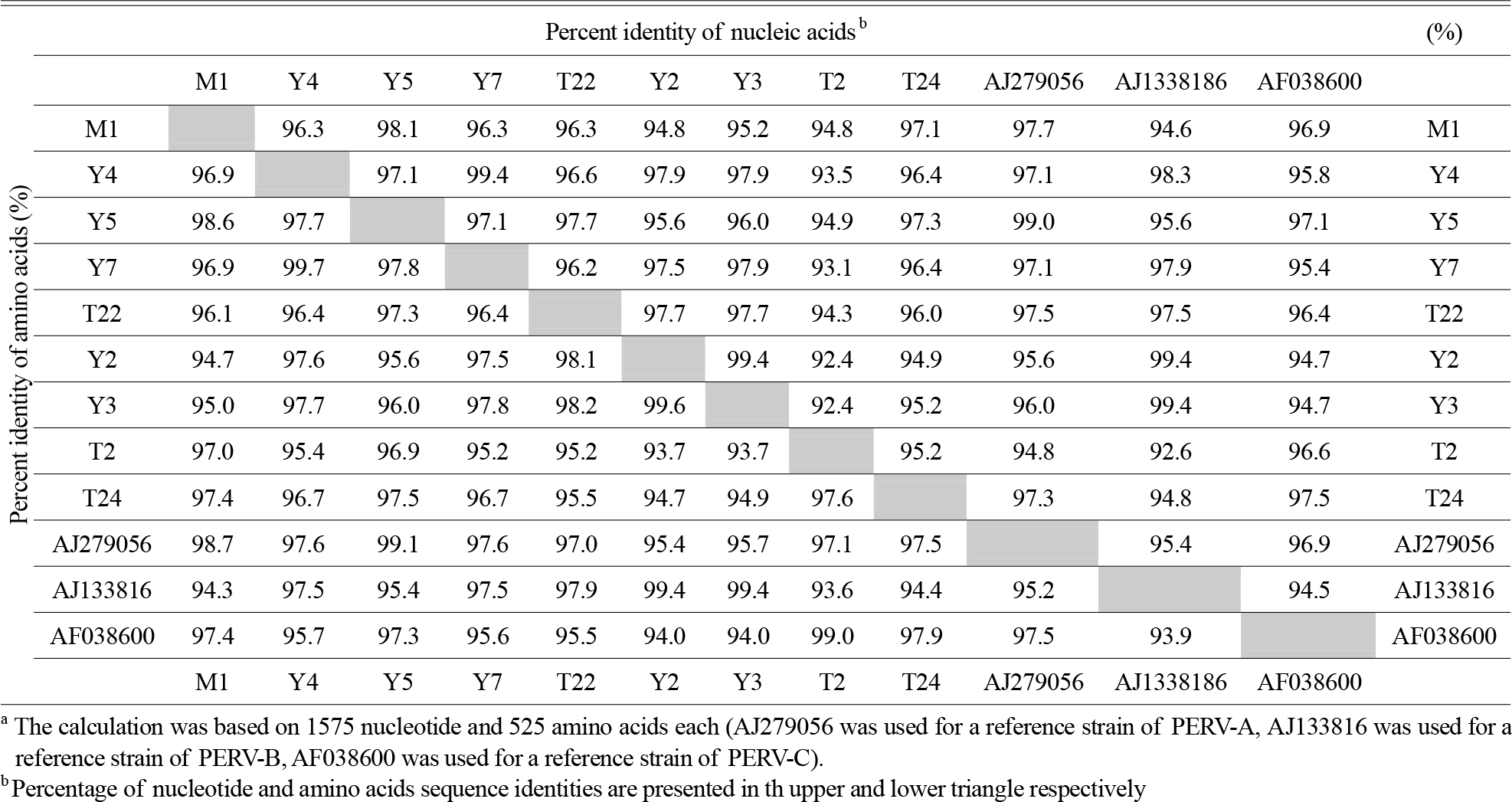
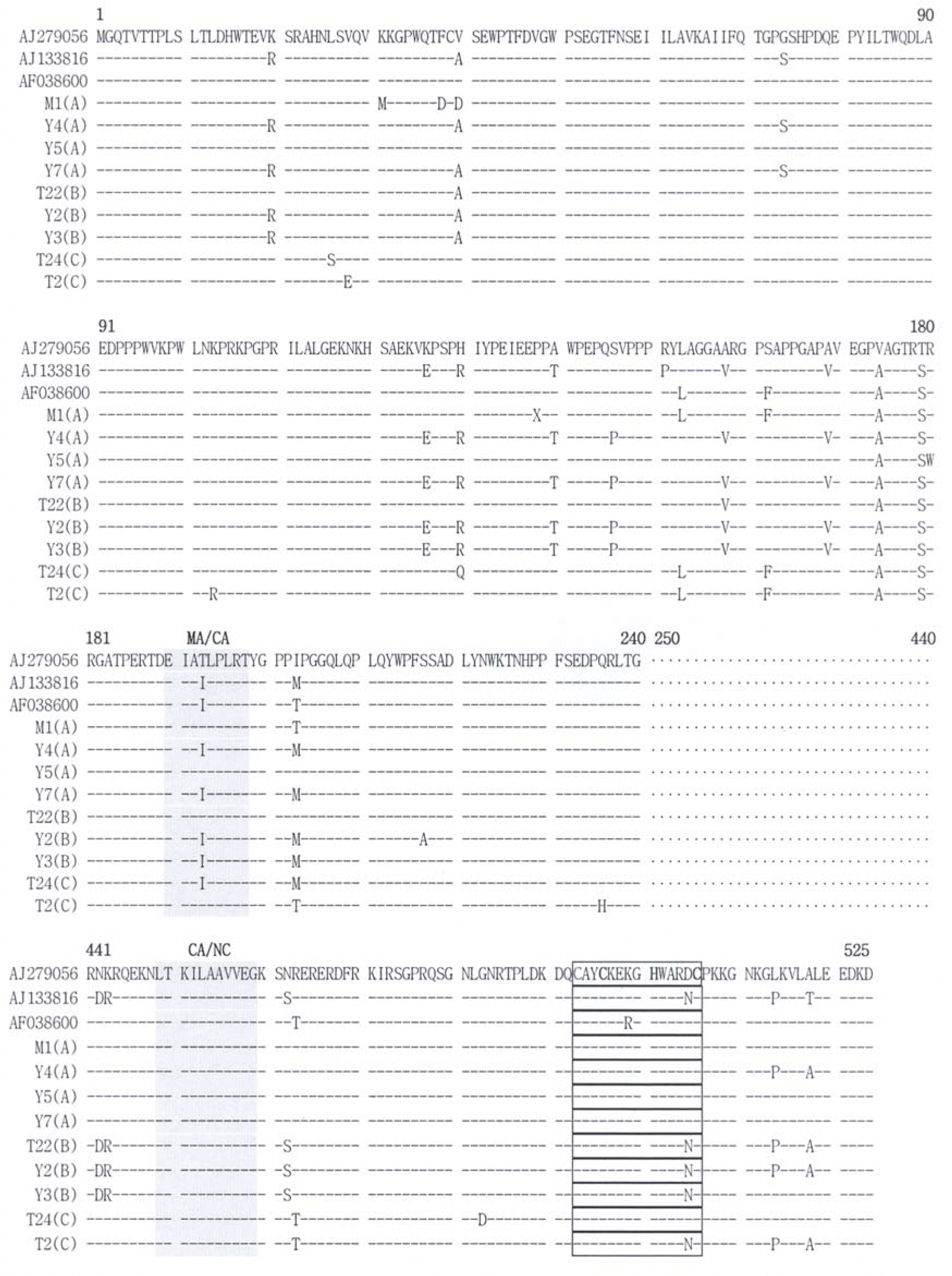 | Figure 2.Alignment of deduced amino acids sequences of PERV Gag. Full length nucleotide sequences of gag clones were identified. Deduced amino acids sequences of cloned gag gene clones were aligned with the reference strains (PERV-A; AJ279056, PERV-B; AJ133816, PERV-C; AF038600). Each type of clones had homology in the nucleotide sequences between 241 and 440. The sequences in shadowed boxes indicate the cleavage sites of Gag, in the closed box indicate Cys-His box. |
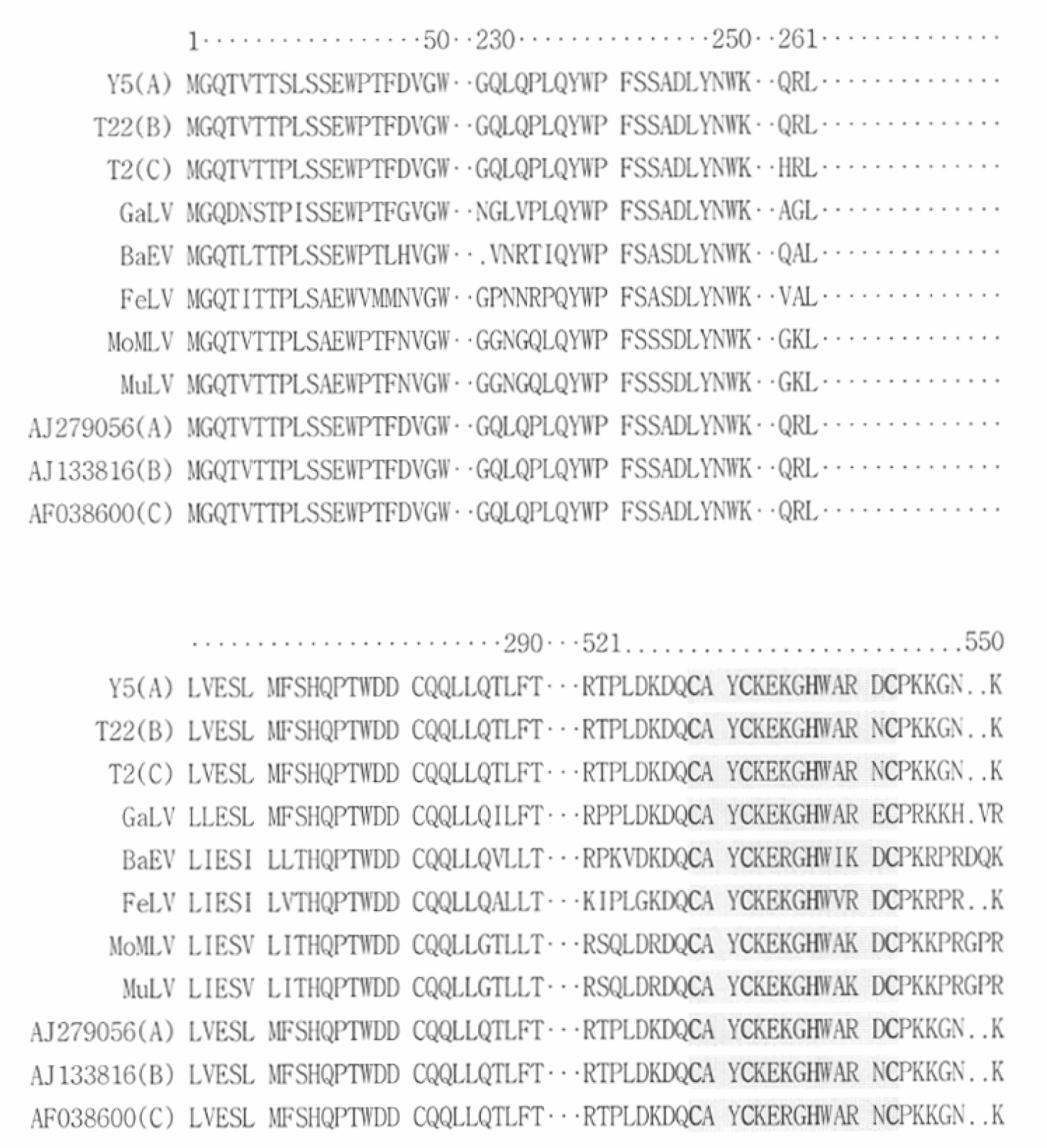 | Figure 3.Comparison of deduced amino acid sequence of PERV gag clones. PERVs (PERV-A: AJ279056, PERV-B: AJ133816, PERV-C: AF038600), GaLV (Gibbon ape leukemia virus, NC001885), BaEV (Baboon endogenous virus, AF142988), FeLV (Feline leukemia virus, K01803), MoMLV (Moloney murine leukemia virus, AF033811), and MuLV (Murine leukemia virus, AB213652) were used as references for alignment of cloned Gag deduced amino acids sequences. Cys-His motif in shadow region, pivotal in virus packaging, was conserved in alignment all gamma-retroviruses. |
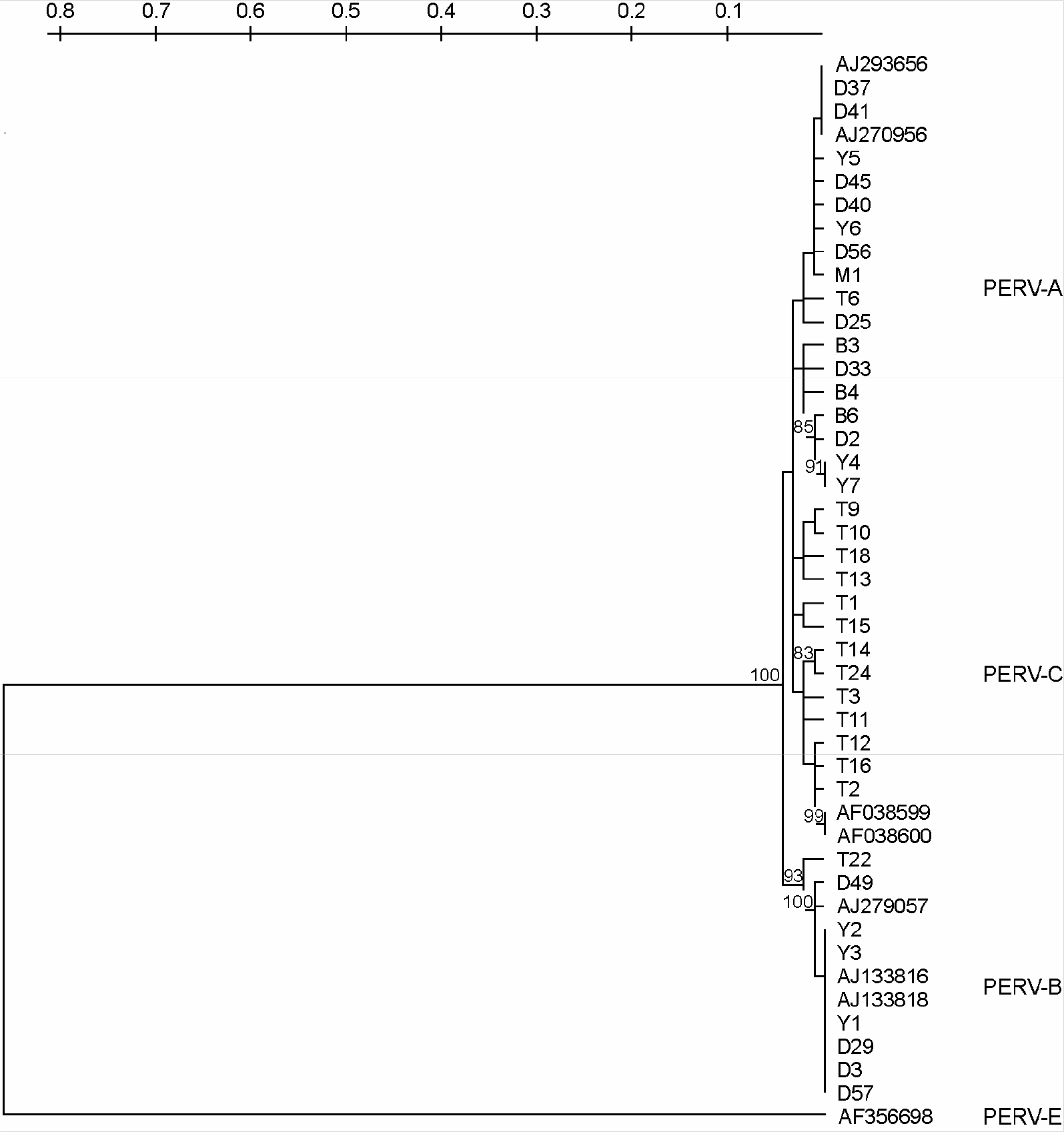 | Figure 4.UPGMA clustering tree based on 1.5 kb nucleotide sequences of PERV gag. The tree was generated by the method of Kimura (1980) on the basis of gag sequences using the Treecon (ver 1.3b.). Numbers at nodes indicate the bootstrap value of 100 resampled datasets. PERV-A (AJ279056 and AJ293656), PERV-B (AJ133816, AJ133818 and AJ279057), PERV-C (AF038599 and AF038600) and PERV-E (AF356698) were used reference strain. Upper scale bar means distances. |
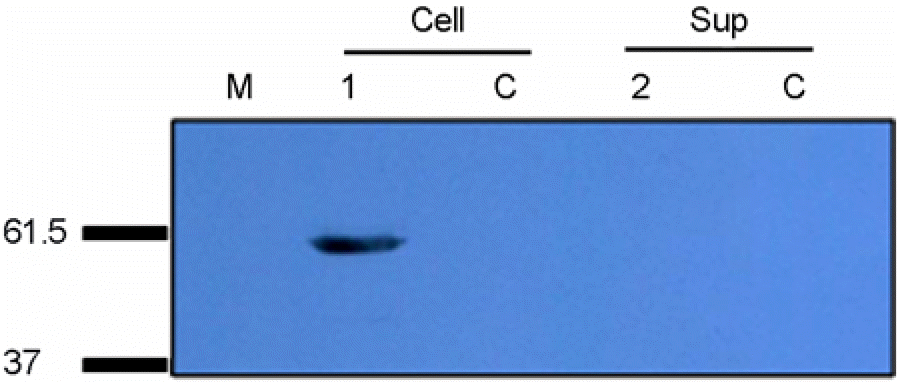 | Figure 5.Immunoblot analysis of expressed PERV Gag protein. Recombinant vaccinia virus harboring PERV gag gene was amplified in HeLa cell. Amplified recombinant vaccinia virus was coinfected with vTF7–3, vaccinia virus producing T7 polymerase, to HeLa cell (lane 1, lane 2). HeLa cell was harvested after 36 hrs later. Detection of expressed PERV Gag protein was performed by anti-His Ab. HeLa cell was used as a control (lane C). The sizes of the molecular weight markers are indicated on the left of panels. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download