Abstract
Coxsackievirus B3 (CVB3) is the nonenveloped virus containing a single-stranded positive-sense RNA as a genome. CVB3 infection can induce acute myocarditis and dilated cardiomypathy. CVB3 of icosahedral symmetry has four capsid proteins called VP1, VP2, VP3, and VP4. Although VP1 is a major antigenic determinant, VP2 is also an important protein for viral physiology, such as maturation cleavage and attenuation. However, VP2 study has been hampered, partly because VP2 antibody is not available. In this study, we developed peptide-based polyclonal VP2 antibody and analyzed its potency by Western blotting analysis and immunofluorescent assay. Purified B3–1 antibody (VP2 peptide antibody developed in here) showed the sensitivity and specificity, similar to VP1 monoclonal antibody which is commercially available. Moreover, this peptide antibody may be useful for double-staining with other antibodies derived from mouse. Therefore, the VP2 antibody may allow us to study CVB assembly and understand VP2 function in depth.
References
1). 전은석. 심근염과 심근증의 원인. 순환기. 23(부록):208–216. 1993.
2). 전은석. 바이러스 심근염과 심근증의 병인기전. 생화학 뉴스. 22(1):36–44. 2002.
3). Ansardi D, Morrow C. Amino acid substitutions in the poliovirus maturation cleavage site affect assembly and result in accumulation of provirion. J Virol. 69:1540–1547. 1995.
4). Appleyard G, Russell SM, Clarke BE, Speller SA, Trowbridge M, Vadolas J. Neutralization epitopes of human rhinovirus type 2. J Gen Virol. 71:1275–1282. 1990.

5). Arnold E, Luo M, Vriend G, Rossmann MG, Palmenberg aCV, Parks GD, Nicklin MJ, Wimmer E. Implications of the picornavirus capsid structure for polyprotein processing. Proc Natl Acad Sci USA. 84:21–25. 1987.

6). Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. Curr Top Microbiol Immunol. 223:31–52. 1997.

7). Basavappa R, Syed R, Flore O, Icenogle JP, Filman DJ, Hogle JM. Role and mechanism of the maturation cleavage of VP0 in poliovirus assembly structure of the empty capsid assembly intermediate at 2.9Å resolution. Protein Sci. 3:1651–1669. 1994.
8). Carpernter CM, Boak RA. Coxsackie viruses; a review of pathologic, epidemiologic, diagnostic and etiologic observations. Calif Med. 77(2):127–130. 1952.
9). Chehadeh W, Lobert PE, Sauter P, Goffard A, Lucas B, Weill J, Vantyghem MC, Alm G, Pigny P, Hober D. Viral protein VP4 is a target of human antibodies enhancing coxsackievirus B4-and B3-induced synthesis of alpha interferon. J Virol. 79(22):13882–13891. 2005.
10). Codd MB, Sugrue DD, Gersh BJ, Melton LJ III. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-cased study in Olmsted County, Minnesota, 1975–1984. Circulation. 80:564–572. 1989.
11). D'Alessio DJ. A case-control study of group B Coxsackie-virus immunoglobulin M antibody prevalence and HLA-DR antigens in newly diagnosed cases of insulin-dependent diabetes mellitus. Am J Epidemiol. 135:1331–1338. 1992.
12). Curry S, Fry E, Blakemore W, Abu Ghazaleh R, Jackson T, King A, Lea S, Newman J, Stuart D. Dissecting the roles of VP0 cleavage and RNA packaging in picornavirus capsid stabilization: the structure of empty capsids of foot-and -mouth disease virus. J Virol. 71:9743–9752. 1997.
13). Dan M, Chantler JK. A genetically engineered attenuated coxsackievirus B3 strain protects mice against lethal infection. J Virol. 79(14):9285–9295. 2005.

14). Fernandez-Tomas C, Baltimore D. Morphogenesis of polio-virus. II. Demonstration of a new intermediate, the provirion. J Virol. 12:1122–1130. 1973.
15). Haarmann CM, Schwimmbeck PL, Mertens T, Schultheiss HP, Strauer BE. Identification of serotype-specific and nonserotype-specific B-cell epitopes of coxsackie B virus using synthetic peptides. Virology. 200:381–389. 1994.

16). Harrison SC. Virus structures and conformational rearrangements. Curr Opin Struct Biol. 5:157–164. 1995.

17). Hindiyeh M, Li QH, Basavappa R, Hogle JM, Chow M. Poliovirus mutants at histidine 195 of VP2 do not cleave VP0 into VP2 and VP4. J Virol. 73(11):9072–9079. 1999.

18). Hogle JM, Chow M, Filman DJ. Three-dimensional structure of poliovirus at 2.9Å resolution. Science. 229:1358–1365. 1985.
19). Kaplan MH, Klein SW, McPhee J, Harper RG. Group B coxsackievirus infections in infants younger than three months of age: A serious childhood illness. Rev Infect Dis. 5:1019–1032. 1983.

20). Kim JY, Jeon ES, Lim BK, Kim SM, Chung SK, Kim JM, Park SI, Jo I, Nam JH. Immunogenicity of a DNA vaccine for coxsackievirus B3 in mice: protective effects of capsid proteins against viral challenge. Vaccine. 23(14):1672–1679. 2005.

21). Kim SM, Park JH, Chung SK, Kim JY, Hwang HY, Chung KC, Jo I, Park SI, Nam JH. Coxsackievirus B3 infection induces cyr61 activation via JNK to mediate cell death. J Virol. 78:13479–13488. 2004.
22). Kitamura N, Semler BL, Rothberg PG, Larsen GR, Adler CJ, Dorner AJ, Emini EA, Hanecak R, Lee JJ, van der Werf S, Anderson CW, Wimmer E. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 291:547–553. 1981.

23). Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 70(11):7811–7818. 1996.

24). Lee WM, Monroe SS, Rueckert RR. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 67:2110–2122. 1993.

25). McLean DM. Coxsackieviruses and echoviruses. Am J Med Sci. 251(3):351–368. 1966.
26). Melnick JL, Hampil B. WHO collaborative studies on enterovirus reference antisera: Fourth report. Bull World Health Organ. 48:381–396. 1973.
27). Minor PD, Ferguson M, Evans DM, Almond JW, Icenogle JP. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 67:1283–1291. 1986.

28). Molla A, Harris KS, Paul AV, Shin SH, Mugavero J, Wimmer E. Stimulation of poliovirus proteinase 3Cpro-related proteolysis by genome-linked protein VPg and its precursor 3AB. J Biol Chem. 269:27015–27020. 1994.
29). Moscufo N, Yafal AG, Rogove A, Hogle J, Chow M. A mutation in VP4 defines a new step in the late stages of cell entry by poliovirus. J Virol. 67:5075–5078. 1993.

30). Muckelbauer JK, Rossmann MG. The structure of coxsackievirus B3. Curr Top Microbiol Immunol. 223:191–208. 1997.

31). Racaniello VR, Baltimore D. Molecular cloning of polio-virus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 78:4887–4891. 1981.

32). Rebel JM, Leendertse CH, Dekker A, Moormann RJ. Effects of mutations in the VP2/VP4 cleavage site of Swine vesicular disease virus on RNA encapsidation and viral infecitivity. Arch Virol. 148:1747–1756. 2003.
33). Rotbart HA, Brennan PJ, Fife KH, Romero JR, Griffin JA, McKinlay MA, Hayden FG. Enterovirus meningitis in adults. Clin Infect Dis. 27:896–898. 1998.

34). Rossamann MG, Johnson JE. Icosagedral RNA virus structure. Annu Rev Biochem. 58:533–573. 1998.
35). Woodruff JF. Viral myocarditis: A review. Am J Pathol. 101:424. 1980.
Figure 1.
CVB3 VP2 region for peptide antibody. (A) Hydrophilicity plot, Antigenic index and Surface probability plot of CVB3 VP2. It derived by DNASTAR programme. (B) Alignment of amino acid sequence of various Enteroviruses, which is used for developing VP2 peptide antibody.
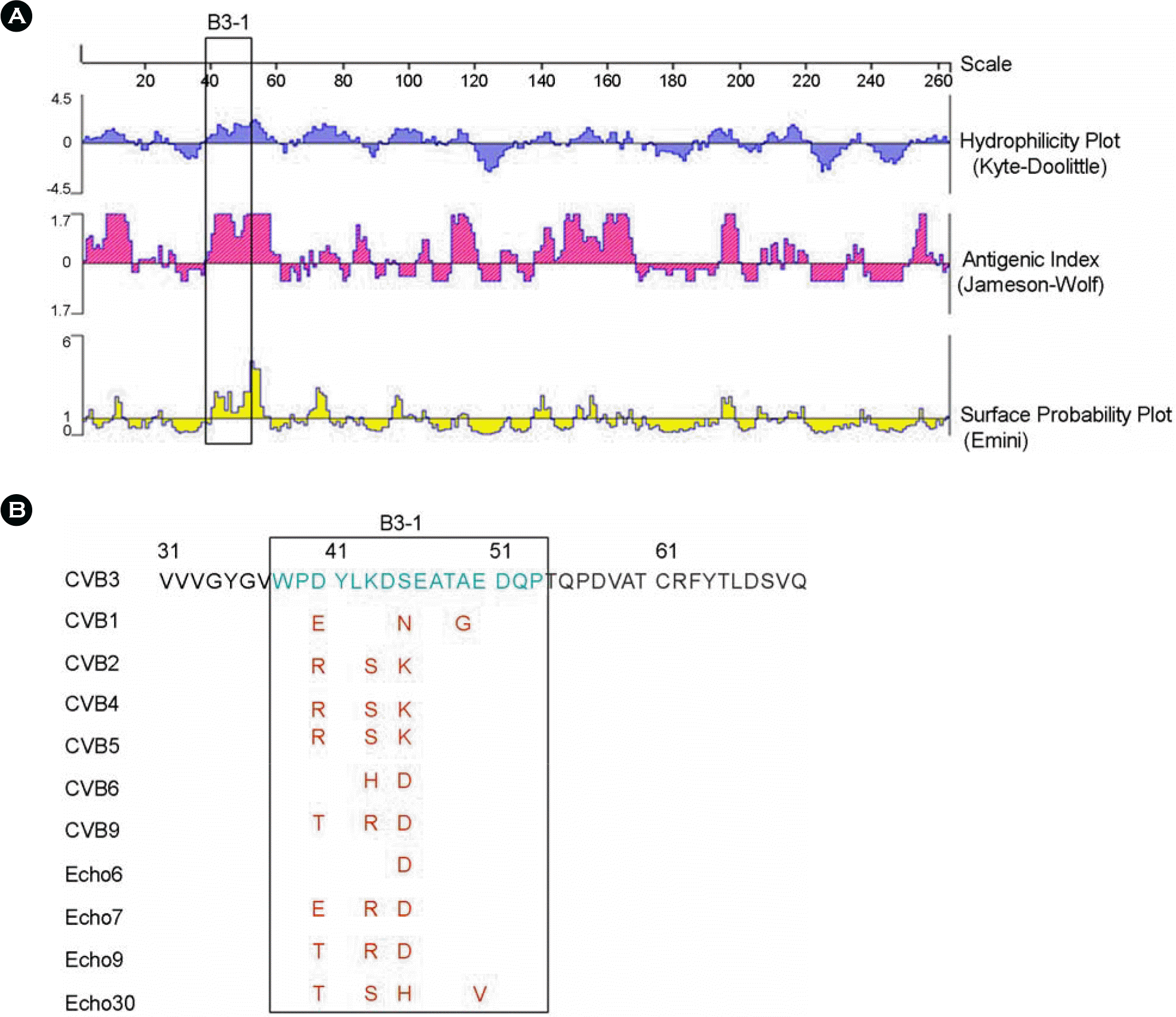
Figure 3.
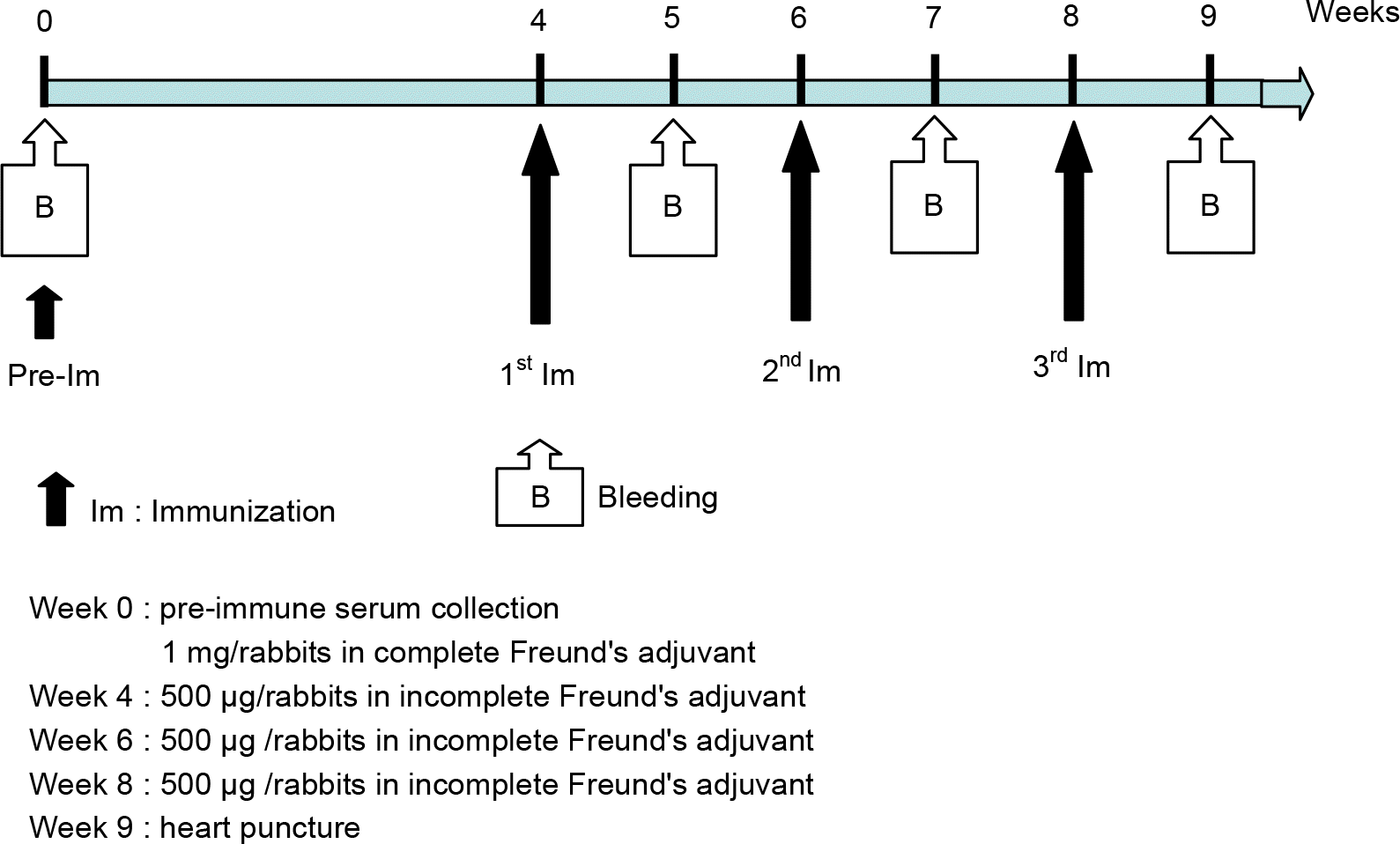
ELISA results from rabbit 1 (A) and rabbit 2 (B). B3-1 peptide was used as antigen and week 9 serum derived from two rabbits immunized with B3–1 peptide immunization were used as primary antibodies.
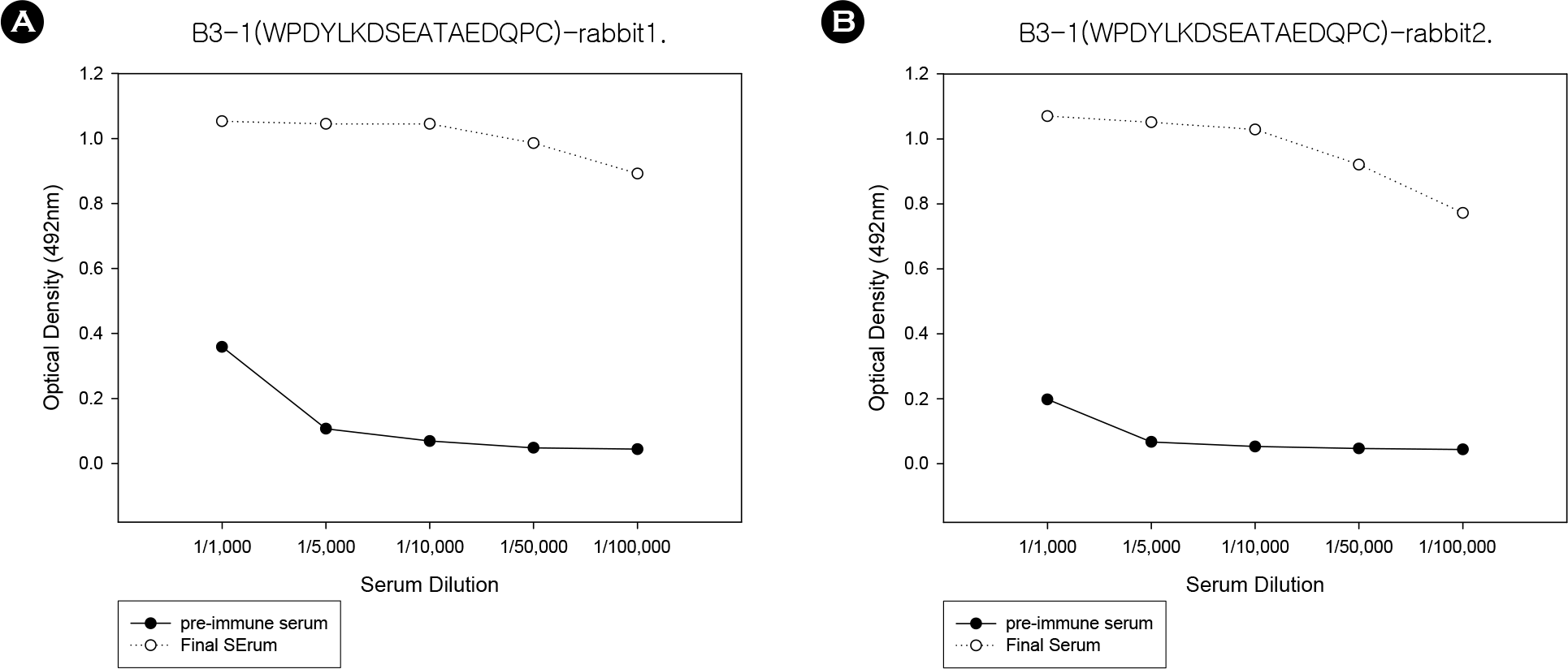
Figure 4.
SDS-PAGE results of purified B3–1 peptide antibody. (A) The primary step is done by processing antigen specific affinity purification. (B) The second step is done by Protein A purification.
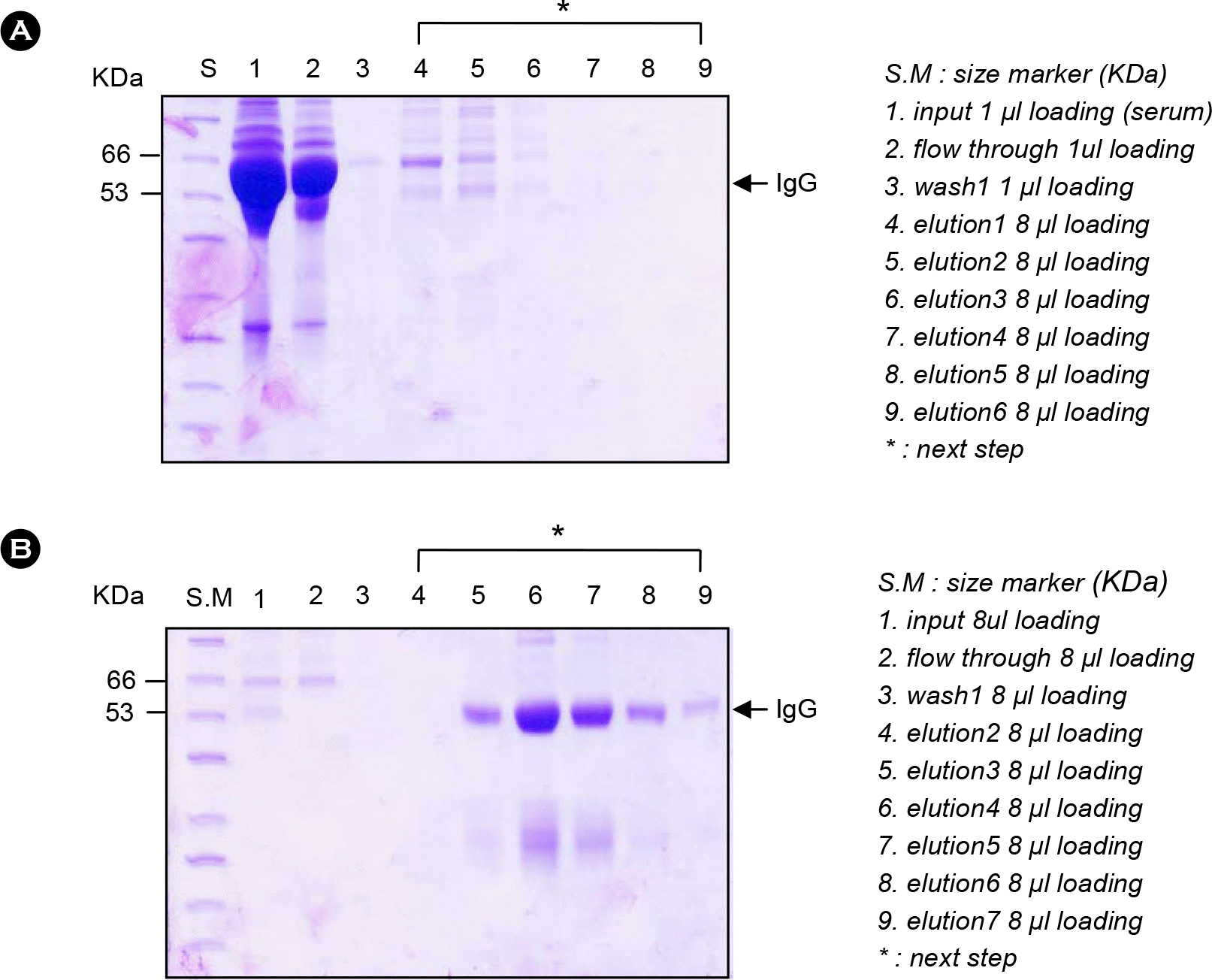
Figure 5.
Comparison of Western blotting results. (A) Various enteroviruses-infected cell lysates are used as antigen for Western blot and purified B3–1 peptide antibody was used as primary antibody. (B) Summary table for Western blot data.
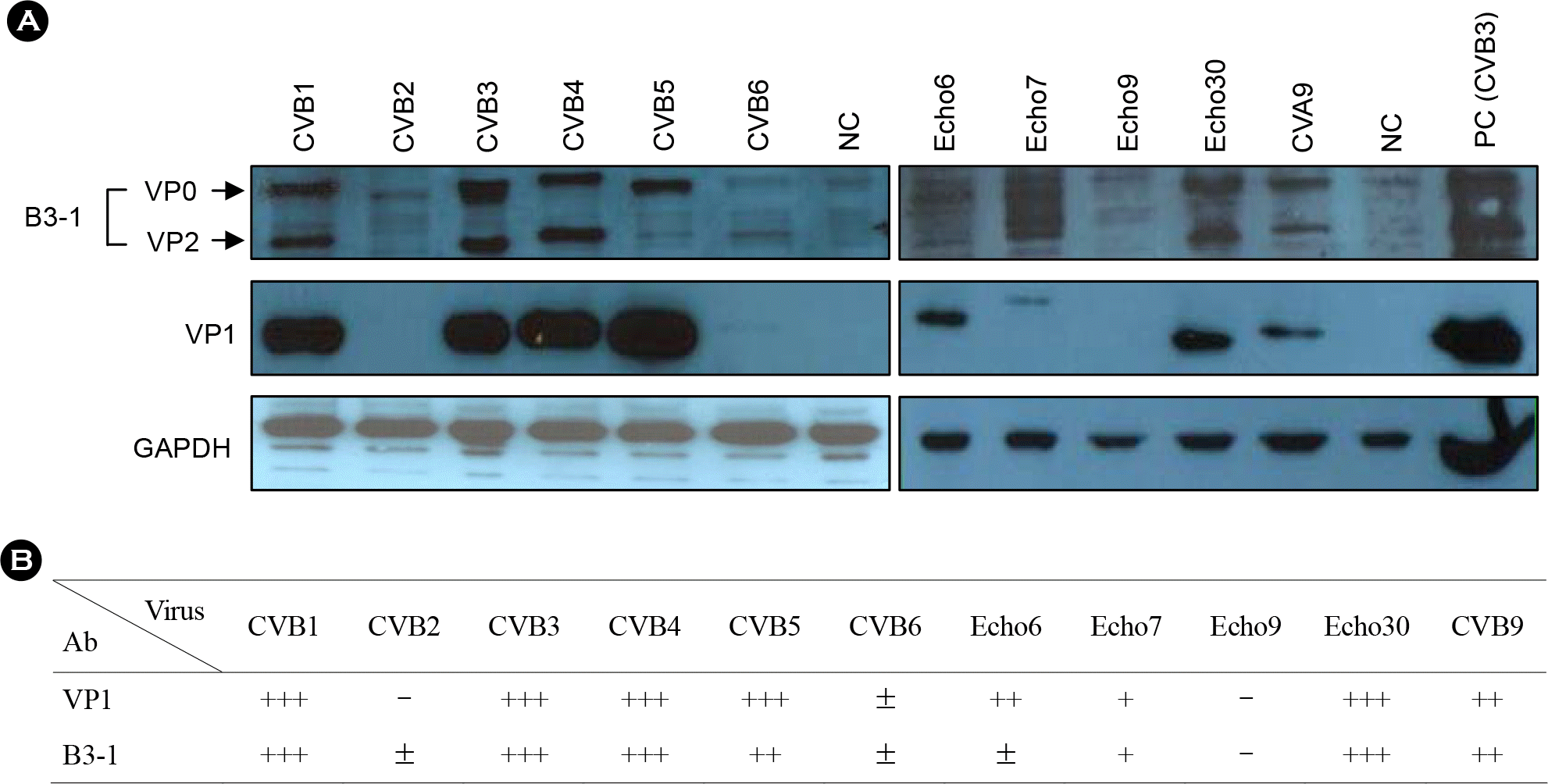
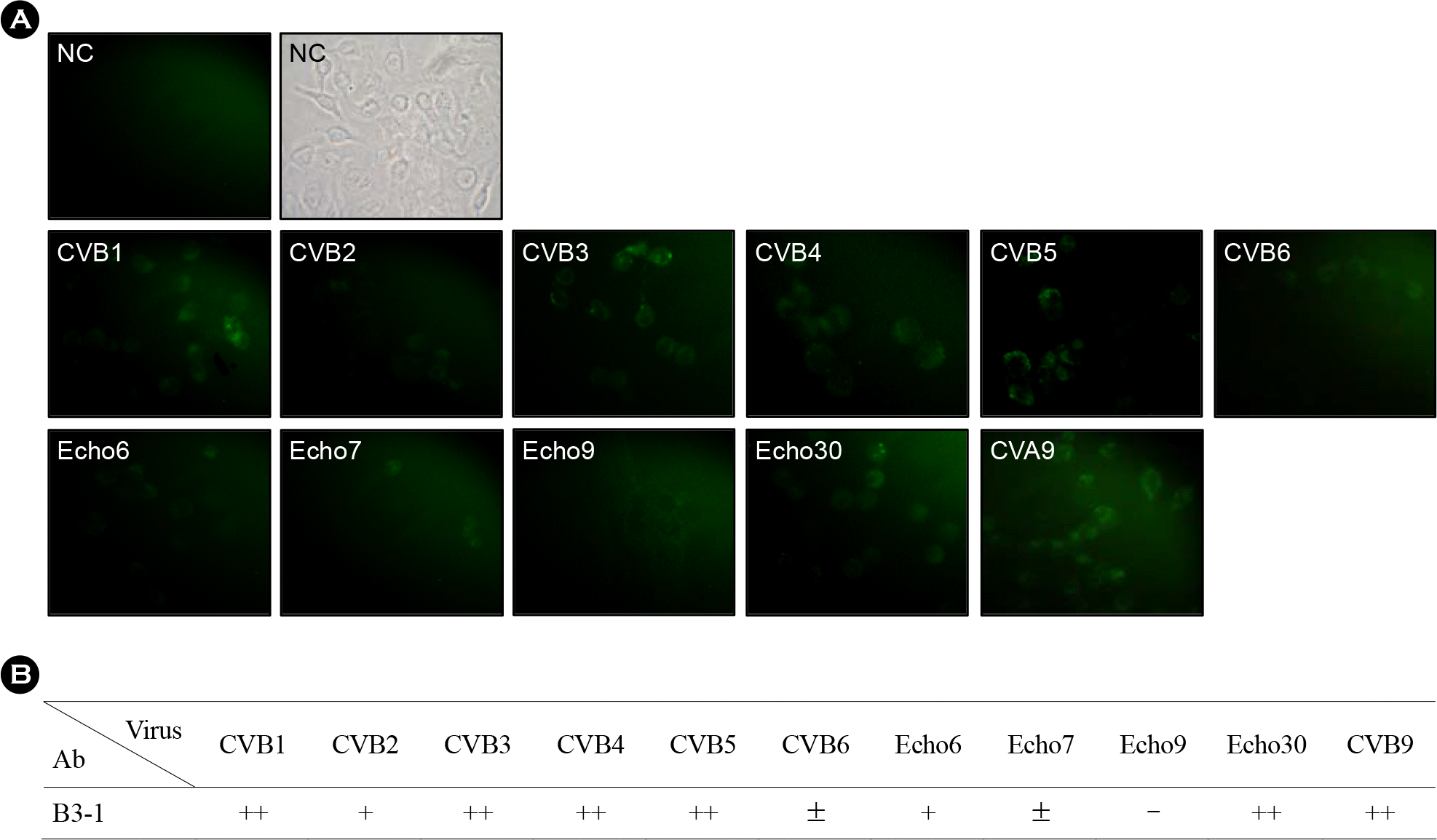
Figure 6.
Comparison of Immunofluorescent assay (IFA) results. (A) Various enteroviruses-infected cells cultured on cover slip are used as antigen for IFA and purified B3–1 peptide antibody was used as primary antibody. (B) Summary table for IFA data. NC indicates negative control which means non-infected cells.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download