INTRODUCTION
TGF-β generates signals through TGF-β receptors (type I and type II serine/threonine kinase receptors) and receptor-regulated Smads (R-Smads) such as Smad2 and Smad3. Either Smad2 or Smad3 is phosphorylated and complexed with Smad4 (1-4). These Smad complexes translocate to the nucleus where they bind specific DNA sequences in target promoters, thereby acting as transcriptional activators for TGF-β-responsive genes. On the other hand, Smad6 and Smad7, termed inhibitory Smads (I-Smads), antagonize TGF-β signaling by inhibiting the phosphorylation of R-Smads (5-7). In addition, TGF-β signaling is regulated by ubiquitin-dependent degradation. First, HECT type E3-ubiquitin ligases such as Smad ubiquitination regulatory factor 1 (Smurf1) and Smurf2 can be recruited to the activated type I receptor (TβRI) by interacting with the Smad7, resulting in receptor ubiquitination and degradation, and reduced signaling (8-10). It has been reported that Smurfs can interact with R-Smad and Runx leading to degradation of these proteins (11-15). Secondly, RING finger type E3 ligase, Arkadia, interacts with Smad7 leading to degradation of Smad7 (16). Third, another HECT type E3 ligase, TG-interacting factor (TGIF) interacting ubiquitin ligase 1 (Tiul1) interacts constitutively with Smad7 and induces degradation of TβRI without affecting level of Smad7 expression. Tiul1 can interact with Smad2/Smad3 and TGIF upon activation of TGF-β1 signaling. The interaction of Tiul1 with TGIF allows this ubiquitin ligase to target Smad2 and Smad3 for degradation, leading to a diminution of TGF-β1 signaling (17). Furthermore, TGIF functions as a negative regulator in TGF-β signaling through recruiting HDACs to a Smad target promoter (18, 19) and inhibition of R-Smads phosphorylation (20).
We have previously shown that TGF-β1, acting mainly through Smad3/4 and Runx3, induces germ-line α (GLα transcription and subsequent class switching recombination (CSR) to IgA, but Smad7 inhibited this TGFβ-induced GLα transcription (21,22). Moreover, we found that Smurfs reinforces the inhibitory effect of Smad7 on TGFβ1-induced IgA CSR while Arkadia antagonizes the action of Smurf causing an increase of IgA CSR (23). In the present study, we provide evidence that Tiul1 and TGIF may be involved in downregulation of TGFβ1-induced IgA isotype expression.
MATERIALS AND METHODS
Cell lines and cell culture
The murine B cell lymphoma line, A20.3 was provided by Dr. J. Stavnezer (University of Massachusetts Medical School, Worcester, MA, USA). CH12F3-2A was provided by Dr. T. Honjo (Kyoto University, Japan). Cells were cultured at 37℃ in a humidified atmosphere containing 5% CO2 in RPMI-1640 medium (Sigma) supplemented with 10% FBS, 50 µM 2-ME, 5 mM HEPES, penicillin (100 U/ml)/streptomycin (100 µg/ml).
Gene expression and reporter constructs
Genes encoding Smad7 (7) subcloned into Flag-pcDNA3 (6) were provided by Dr. M. Kawabata (The Cancer Institute, Tokyo, Japan). For Tiul1 expression constructs, the open reading frame was amplified from the γ-ZAP11 clone by PCR and subcloned into p3xFlag-CMV-10 expression vector (Sigma). A similar approach was used to clone TGIF fragments in pcDNA3-HA (17).
RT-PCR
RNA preparation, reverse transcription, and PCR were performed as described previously (21). Primers for PCR were synthesized by Bioneer Corp. (Seoul, Korea). The primers for GLTα were: forward primer, 5'-CTACC ATAGG GAAGA TAGCC T-3', and reverse primer, 5'-TAATC GTGAA TCAGG CAG-3' (product size, 267 bp); PSTα were: forward primer, 5'-CTCTG GCCCT GCTTA TTGTT G-3', and reverse primer, 5'-GAGCT GGTGG GAGTG TCAGT G-3' (product size, 267 bp); CTα were: forward primer, 5'-CTACC ATAGG GAAGA TAGCC T-3', and reverse primer, 5'-TCTGA ACCTT CAAGG ATGCT CTTG-3' (product size, 365 bp); GLTγ3 were: forward primer, 5'-CAAGT GGATC TGAAC ACA-3', and reverse primer, 5'-GGCTC CATAG TTCCA TT-3' (product size, 349 bp); β-actin were: forward primer, 5'-CATGT TTGAG ACCTT CAACA CCCC-3', and reverse primer, 5'-GCCAT CTCCT GCTCG AAGTC TAG-3' (product size, 320 bp). All reagents for RT-PCR were purchased from Promega Corp. PCR reactions for β-actin were performed in parallel in order to normalize cDNA concentrations within each set of samples. Aliquots of the PCR products were resolved by electrophoresis on 2% agarose gels.
Isotype-specific ELISA
ELISAs were performed as described previously (21). The reaction products were measured at 405 nm with an ELISA reader (VERSAMAX reader, Molecular Devices, Sunnyvale, CA).
RESULTS AND DISCUSSION
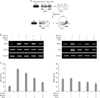
Tiul1 and TGIF inhibit TGFβ1-induced GLα promoter activity
Although Tiul1 down-regulates TGF-β signaling by inducing degradation of the activated type I receptor and R-Smads (17), it is not known if Tiul1 is involved in TGFβ1-induced IgA CSR. Therein, we investigated the effect of Tiul1 on TGFβ1-induced GLα transcription in A20.3 B lymphoma cell lines, using a GLα promoter reporter. As shown in Fig. 1A, over-expression of Tiul1 decreased promoter activity by twofold. In addition, it strengthened the inhibitory effect of Smad7 on the promoter activity (Fig. 1A). TGIF down-regulates TGF-β signaling through recruiting HDACs to a Smad target promoter (18,19) and inhibiting R-Smads phosphorylation (20). Further, TGIF interacts with Tiul1 in the nucleus leading to the degradation of R-Smads (17). We tested the effects TGIF along with Tiul1 on TGF-β induced GLα promoter activity. As shown in Fig. 1B, overexpression of TGIF decreased the TGF-β induced GLα promoter activity. Moreover, TGIF strengthened the inhibitory effect of Tiul1 on the promoter activity. Taken together, these results suggest that Tiul1 not only interacts with Smad7 but also with TGIF, both of which lead to the downregulation of GLα gene expression.
Effect of Smad7 and Tiul1 on the expression of endogenous IgA transcripts and IgA secretion
Thus far, we observed that Tiul1 acts as the negative regulator in TGFβ1-induced GLα promoter activity. To gain evidence that this phenomenon is physiologically relevant, we asked if Tiul1 actually inhibits the expression of transcripts associated with IgA CSR. As shown in the diagram in Fig. 2A, once CSR to IgA occurs, the GLµ promoter, which becomes associated with the Cα gene and continues to be active, generates transcripts termed α post-switch transcripts (PSTα) (24,25). Furthermore, the DNA sequences between Sµ and Sα are looped out of the chromosome as switch circles during CSR, and another type of transcript, termed a circle transcript (CT), in this case consisting of the Iα exon spliced to the Cµ exon (CTα), is transcribed from the switch circle owing to the presence of the active Iα promoter (26). Thus, expression of PSTα and CTα as well as GLTα can be used as indicatives of active IgA CSR. As in the case of the GLα promoter reporter, overexpression of Smad7 decreased TGFβ1-induced GLTα expression (Fig. 2B). In this, Tiul1 again strengthened the inhibitory effect of Smad7 on the GLα transcription, but not GLTγ3. Similarly, Smad7 and Tiul1 in combination downregulated the expression of PSTα and CTα. Finally, we examined the effects of Smad7 and Tiul1 on IgA secretion. As shown in Fig. 2C, over-expression of either Smad7 or Tiul1 alone decreased TGFβ1-induced IgA secretion, and the combination markedly diminished IgA secretion. Not addressed specifically, these results implicate that Tiul1 degrades TβR1 through interacting with Smad7 as shown before (17), resulting in reduction of TGFβ1-induced IgA production.
Effect of Tiul1 and TGIF on TGFβ1-induced IgA expression
Since we observed that Tiul1 in cooperation with Smad7 downregulate TGF-β induced IgA expression, we examined if Tiul1 together with TGIF can also regulate TGFβ1-induced IgA expression. As shown in Fig. 3A, either overexpression of Tiul1 or TGIF decreased the TGFβ1-induced GLTα transcription. Overexpression of both molecules more dramatically decreased the expression of GLTα but not GLTγ3. In fact, this was the case for the TGFβ1-induced IgA secretion (Fig. 3B). These results indicate the possibility that Tiul1, in cooperation with TGIF, can inhibit TGFβ1-induced IgA production.
Concluding remarks
In the present study, we have shown that Tiul1 and TGIF can down-regulate TGFβ1-induced IgA CSR. Possible mechanisms underlying this phenomenon are illustrated in Fig. 4. In this model, Tiul1 downregulates TGFβ1-induced IgA CSR through degradation of activated TβR-I. Secondly, TGIF inhibits TGFβ1-induced IgA CSR by the inhibition of R-Smads. Third, Tiul1 along with TGIF decreased TGFβ1-induced IgA CSR via degradation of R-Smads such as Smad2 and Smad3. Since we observed in the present study that Tiul1 can act a negative regulator in association with Smad7 and TGIF toward TGFβ1-induced IgA CSR, it would be important to elucidate the dynamics of interrelation among Smad7, Tiul1, and TGIF along with Smurfs (23) in the context of TGFβ1-induced IgA expression in the future.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download