Abstract
Background
Melanoma is the most fatal form of skin cancer due to its rapid metastasis. Recently, several studies reported that selenium can induce apoptosis in melanoma cells. However, the precise mechanism remains to be elucidated. In this study, we investigated the effect of selenium on cell proliferation in murine melanoma and on tumor growth and metastasis in C57BL/6 mice.
Methods
Cell proliferation was measured by MTT assay in selenium-treated melanoma cells. Cell cycle distribution was analysized by staining DNA with propidum iodide (PI). mRNA and protein expression related to cell cycle arrest was measured by reverse transcription PCR and western blot. Tumor growth and metastasis was measured by in vivo model.
Results
Selenium was suppressed the proliferation of melanoma cells in a dose dependent manner. The growth inhibition of melanoma by selenium was associated with an arrest of cell cycle distribution at G0/G1 stage. The mRNA and protein level of CDK2/CDK4 was suppressed by treatment with selenium in a time-dependent manner. In vivo, tumor growth was not suppressed by selenium; however tumor metastasis was suppressed by selenium in mouse model.
Malignant melanoma is the most aggressive skin cancer due to its capacity to rapidly metastasize and strong resistance to anti-cancer therapy, such as chemotherapy and radiotherapy (1). In spite of extensively researched melanoma physiology, the associated rate of mortality is high and critical therapeutic approaches are still not sufficient. Several reports showed that treatment with antioxidants is more beneficial in preventing the rapid growth and metastasis of melanoma cells (2,3). In particular, the combination of anti-cancer drug and anti-oxidant is more effective than single treatment because the combination treatment leads to overall low toxicity via synergistic effect (4).
Selenium is an essential trace element involved in cellular homeostasis and protection against cellular stress such as ROS (reactive oxygen species) and DNA damage (5). Patrick et al., report that selenium has a function as an anti-cancer agent (6). Several reports demonstrate that sodium selenite, a source of selenium in many food supplements, can strongly induce cell death in various cancer cells (7-9). Additionally, recent studies reveal that sodium selenite display effective anti-cancer activity against melanoma cancer cells. Despite much evidence about the anti-cancer and anti-oxidant effects of selenium, the exact mechanism whereby this chemical induces cell death in various cancer cells is not yet understand.
Recent studies revealed that ROS play a pivotal role in selenium-mediated cancer cell death (10-12). The ROS level increase cell death following treatment with selenium by inducing DNA breakage and cell cycle suppression. It is well known that the CDK (cyclin-dependent kinase) has a critical role in ROS related cell cycle regulation. Some studies report that activation of p53 is induced by CDK2 gene expression upregulated by treatment with selenium, which then induces cell death in mouse mammalian epithelial carcinoma cell line. Moreover, activation of p53 and p38 pathway has been shown to induce apoptosis in human cervical cancer following treatment with selenium (13-16). Although the effects of selenium on the growth and metastasis of some cancer cells are well known, the anti-cancer effects of selenium on cancer cell growth and cell migration in melanoma are not completely understood. In this study, we evaluate the anti-cancer effects of selenium on a murine melanoma cell line and the anti-metastatic ability of selenium on an experimental metastasis model using B16 mouse melanoma cells. These findings provide useful information on the therapeutic tool of antioxidant treatment metastatic melanoma cancer.
B16F10, murine melanoma cell line, was maintained in RPMI 1640 supplemented with 2 mM L-glutamine, 100 units/ml penicillin, 100 ug/ml streptomycin and 10% heat-inactivated fetal bovine serum. The cells were cultured at 37℃ in a humidified atmosphere that contained 5% CO2. These cells were used for experiments while they were in the log phase of growth. Sodium selenite (selenium) was purchased from Sigma (St. Louis, MO, USA).
The inhibition of cell growth was determined by 3 (4, 5-dimethyl thiazolyl-2) 2, 5 diphenyl tetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) assay. B16F10 mouse melanoma cells were seeded on 96 well plates (1×103 cells/well) and treated with 0, 1, 2, 10, 25, 50, or 100 uM selenium for 72 hrs before adding 20 ul MTT solution (0.5 mg/ml). The MTT absorbance was measured at 570 nm using an ELISA reader (Bio-Tek instruments, Inc., Winnoski, VT, USA) 4 h after addition of the dye. Each experiment was performed in triplicate.
To investigate the apoptosis-inducing effect of selenium, cells were collected, washed twice with PBS, and then resuspended in 100 ul of Annexin V binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). After 2 ul of FITC-conjugated Annexin V (BD Pharmingen, San Diego, CA, USA) and 2 ul of propidium iodide (BD Pharmingen) were added, cells were incubated in the a dark room at room temperature for 15 minutes with gentle shaking. Finally, 400 ul of Annexin V binding buffer was added to each tube and cells were analyzed using a FACSCalibur (BD Pharmingen).
Cell cycle analysis was performed using propidium iodide (PI)-staining method. Cells were harvested after selenite treatment, washed twice in PBS (2% FBS), and fixed with 70% cold aqueous ethanol at 4℃ overnight. The cells were resuspended in a PI stain (10 ug/ml RNase A and 10 ug/ml PI in PBS) at room temperature for 30 minutes, and DNA content was determined using a FACSCalibur (BD Pharmingen).
To assess the effect of selenite on cell cycle regulatory gene expression, the cells were harvested after selenite treatment and washed twice in cold PBS. RNA was extracted using an RNeasy mini kit (Quiagen, Valencia, CA) according to the manufacturer's instructions, and RT premix (Bioneer, Daejeon, South Korea) was used to transcribe cDNA. RT-PCR analysis of specific gene transcript was carried out using the following primers for p53 (upstream primer; 5'-ATG ACT GCC ATG GAG GAG T, downstream primer; 5'-CTC GGG TGG CTC ATA AGG TA, 664-bp product), p21Waf1 (upstream primer; 5'-CTT TGA CTT CGT CAC GGA GAC, downstream primer; 5'-AGG CAG CGT GAA GGT GTT TGG GG, 480-bp product), CDK2 (upstream primer; 5'-CAC AGC CGT GGA TAT CTG GAG, downstream primer; 5'-TTG CGA TAA CAG GCT CCG TC, 253-bp product), CDK4 (upstream primer; 5'-ACG CCT GTG GTG GTT ACG CT, downstream primer; 5'-CCA TCT CTG GCA CCA CTG AC, 280-bp product). β-actin (upstream primer; 5'-AAG AGC TAT GAG CTG CCT GA, downstream primer; 5'-CAG GAG GAG CAA TGA TCT TG, 300-bp product) was used as an internal control. PCR (24cycle; 30 seconds at 94℃, 30 seconds at 60℃, 60 seconds at 72℃) was performed using AccuPower PCR premix (Bioneer).
Cells (1×107 cells/sample) were scraped into cold PBS, centrifuged and the lysed in RIPA buffer (EIPiS, Daejeon, South Korea) on ice for 30 min. Each cell lysate was separated by 15% SDS-PAGE under reducing conditions and transferred to a nitrocellulose membranes using semidry technique at 80mA for 2 hours. The blots were blocked with 5% skim milk in PBS for 1 hour, and subsequently incubated with primary antibodies (Abs) at a final dilution of 1:1,000 for 2 hour. After three washes in PBST (0.05% tween-20 in PBS), blot were incubated with peroxidase-conjugated secondary antibodies (final dilution, 1:3,000) in dilution buffer (5% skim milk in PBS) for 1 hour and then washed as described above. Detection was performed by chemiluminescence using an ECL-kit (Enhanced Chemi Luminescence; Amersham Life Science, Braunschweig, Germany) and the Multiple Gel-DOC system (Fujifilm, Tokyo, Japan). The following primary antibodies were used: p21 Waf1/Cip1, CDK4, CDK2 and β-actin from Cell Signaling (Beverly, MA, USA).
Six- to 8-week-old C57BL/6 mice were obtained from Orient (Seoul, Korea). The institutional review board of animal experiments reviewed all animal studies. Six- to 8-week-old C57BL/6 mice were injected with 0.1 ml of PBS containing 0.1 million B16F10 cells by intra-optic venous route. Mice were randomly assigned to 5 groups (5 mice/group). The mice were treated from day 1 onward with 10 uM selenium through intraperitoneal route. On days 4 and 8 the mice received selenium in 200 µl PBS injected i.p. The mice were killed on day 14, and melanotic colonies on the surface of the lung were counted. The lung sections were fixed, stained with hematotoxylin-eosin (H&E) and was observed under a phase contrast microscope.
To assess whether selenium directly induces cell death of B16F10 melanoma cells, cells were treated with 0, 1, 2, 10, 25, 50, or 100 uM of selenium. Selenium-induced inhibition of cell growth was determined by MTT assay. Fig. 1A shows that the growth of cells exposed to 50 uM selenium for 72 hour was significantly reduced to 35% of control group. We also found that selenium inhibited the growth of cells in a dose-dependent manner. To confirm our result, we observed the morphological changes of B16F10 cells exposed to 40 uM selenium at 24 hours and 48 hours. As shown in Fig. 1B, treatment with 40 uM selenium significant decreased the cell number of B16F10 melanoma cells in a time-dependent manner.
Next, we measured apoptosis of B16F10 cells by flow-cytometry analysis. Cell cultures were treated under various conditions for 72 hours. Fig. 2A shows that treatment with selenium induces apoptosis in B16F10. The number of apoptotic cells following treatment with 40 uM selenium was significantly higher as compared with the low-dose treated group.
To determine the mechanism of selenium-induced apoptosis, we analyzed the cell cycle of selenium treated B16F10 by PI staining method. Addition of sodium selenium (40 uM) significantly increased the G0/G1 arrest in B16F10 cells compared with the low-dose treated group (Fig. 2B). Taken together, these results indicate that G0/G1 cell cycle arrest is dominantly involved in selenium-induced apoptosis.
In order to examine the mechanism of selenium-induced G0/G1 arrest in B16F10 cells, we investigated the mRNA and protein expression pattern of various cell cycle regulators such as p53, p21Waf1, CDK2 and CDK4 after treatment with selenium. As shown in Fig. 3A, treatment with selenium significantly decreased expression of p53, CDK2 and CDK4 mRNA in time-dependent manner. However, p21Waf1 mRNA was increased in a time-dependent manner following treatment (Fig. 3A). Furthermore, after exposing cells to 40 uM selenium, the protein level of p21WAF1 significantly increased, while protein levels of CDK2 and CDK4 decreased in time-dependent manners (Fig. 3B). This result implies that selenium-induced cell cycle arrest is regulated by a p53-independent p21Waf1 pathway.
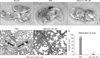
Treatment with selenium regulated p53-independent pathway and cell growth, which are both related to tumor cell growth. To evaluate the effect of selenium on melanoma cell growth, the in vivo growth of melanoma cells in C57BL/6 mouse was determined after the subcutaneous inoculation of B16F10 cells and treatment with selenium. When selenium was administered syngeneic mice, severe regression of tumor growth was not observed (Fig. 4). However, B16F10 melanoma-bearing mice treated with selenium had a significantly reduced tumor burden as measured by counting the number of lung colonies (Fig. 5A). Additionally, we tested for lung metastatic tumor cells to B16F10 bearing mice using histochemical H&E staing. As shown in Fig. 5B and C, treatment with selenium significant decreased the number of lung metastatic spots by B16F10 melanoma cells. These results demonstrate that selenium promotes anti-cancer activity and anti-metastasis activity in melanoma cell bearing mice.
One of the most important problems with removing melanoma is its powerful metastatic tumor growth. In the present study, we have evaluated the effect of selenium on controlling B16F10 melanoma proliferation and metastasis. It is well known that anti-oxidant compounds, such as ascorbic acid and selenium, have the ability to inhibit cancer growth and metastasis (2,3). Selenium compound has been specifically reported to significantly reduce cell proliferation and incidence of cancer in vivo by being added to in vitro cell culture or supplemented in animal diets (6). However, the detailed mechanism of its anti-cancer and anti-metastatic effects remains poorly understood.
We evaluated the effects of selenium on the lung metastasis of B16F10 melanoma cells in vivo. Interestingly, our results show that the selenium-treated group had reduced metastasis of B16F10 melanoma cells, although treatment with selenium did not reduce the tumorigenesity of B16F10 melanoma cells (Fig. 4, 5). To identify the affects of selenium on the relationship of tumorigenesity and metastasis in B16F10 melanoma cells, treated cells were analyzed for changes in cell morphology and apoptosis (Fig. 1, 2). Treatment with selenium led to morphologic changes in B16F10 melanoma cells and increase in apoptotic events in a dose-dependent manner. These results suggest that significant anti-metastatic activity of sodium selenite may be due to the apoptotic activity of selenium.
More interestingly, our results revealed that cell apoptosis of B16F10 melanoma cells mediated G0/G1 arrest following treatment with selenium. This is significant because it is well known that regulation of the cancer cell cycle is one of the therapeutic targets for identification of new anti-cancer strategy. Of particular importance are disruptions of cell cycle at the G0/G1 check-point, which leads to dis-regulation of tumor growth (17-21). Based on this evidence, we focused on the mechanism of G0/G1 cell cycle arrest in selenium-induced apoptosis. Many studies have reported that p53 is involved in G0/G1 cell cycle arrest (22-25). However, Deeds et al. recently observed that G0/G1 arrest is induced in a p53-independent manner (26). As shown in Fig. 3, our results support the findings that selenium induces G0/G1 arrest via p53-independent pathway through expression of p21Waf1. Several studies have revealed that p21WAF1 has a critical role in the mechanism of G0/G1 arrest, which involves association of CDK-cyclin complex to inhibit CDK kinase activity (27). We found that treatment of melanoma with sodium selenium induces expression of p21WAF1, which leads to down-regulation of CDK4 and CDK2 expression at the mRNA and protein levels (Fig. 3). Our findings suggest that selenium induces cell apoptosis due to G0/G1 arrest through a p53-independent p21WAF1 pathway.
In conclusion, we suggest that treatment with selenium enhances the ability of anti-metastasis to promote apoptosis of B16F10 melanoma cells by inducing p53-independent G0/G1 cell cycle arrest. Further knowledge regarding the treatment of selenium may provide a usefully concept for therapy strategy to improve malignancy in cancer patients.
Figures and Tables
Figure 1
Effect of selenium on cell viability and morphological changes of B16F10 murine melanoma cells. (A) Selenium inhibits cell viability of B16F10 murine melanoma cells (MTT assay). B16F10 melanoma cells (1×103) were seeded onto a 96-well plate and treated with various doses (0. 1, 5, 10, 25, 50 and 100 uM) of selenium for 72 hr. MTT solution (100 ug/ well) was added to the cells 4 hr later. (B) B16F10 cells (2×105) were seeded onto a 6-well plate overnight to undergo attachment and then treated with various doses (0, 10, 20, and 40 uM) of selenium. After 48 h, photos of cells were taken with an inverted microscope.
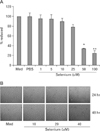
Figure 2
Selenium-induced cell death and cell cycle arrest of B16F10 murine melanoma cells. (A) B16F10 melanoma cells (2×105) were seeded onto a 6-well plate overnight to undergo attachment. Cells were exposed to selenium (0, 10, 20, and 40 uM) and then incubated for 48 hr. After incubation, cells were harvested with cell dissociation solution and washed once with PBS. The cytotoxic effect of selenium on B16F10 melanoma cells was assessed by Annexin-V-FITC/PI staining. Cell death analysis was performed by flow cytometry. The numbers indicate the percentages of the viable (Annexin-V -; PI -) cell population (lower left quadrant), the apoptotic (Annexin V+) cell population (lower right quadrant), and the necrotic cell populations in the upper right (Annexin-V +; PI +) and the upper left quadrants (Annexin-V -; PI +). (B) B16F10 melanoma cells were treated with selenium (0, 10, 20, and 40 uM) and then incubated for 48 hr. Cells were harvested with cell dissociation solution, washed twice with cold PBS, and centrifuged. The pellet was resuspended in 1 ml of cold PBS and 4 ml of cold ethanol for 30 min at 4℃. Cells were then stained with propidium iodide 30 min and analyzed by flow cytometry. Percentages of G0/G1-, S-, and G2/M-phase cells are shown in this figure.

Figure 3
The effect of selenium on the expression of cell cycle arrest related genes in B16F10 melanoma cells. (A) B16F10 melanoma cells were harvested at the indicated times after incubation with selenium (40 uM). Total RNA was extracted from cells at 48 hr and cDNA was prepared using methods described in the Material and Methods section. (B) B16F10 melanoma cells were harvested at the indicated times after incubation with selenium (40 uM). The cells were then lysed, and the supernatants were subjected to western blot analysis and immunoblotted with anti-p21Waf1/Cip1, anti-CDK4, and anti-CDK2 antibodies.
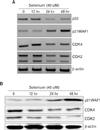
Figure 4
The effect of selenium on tumor growth. B16F10 melanoma cells were injected s.c. into mice. Selenium was injected at the tumor site. Selenium was then injected 2 more times in 4-day-period. Tumor size was measured every other day by a vernier calipers.

Figure 5
The effect of selenium on metastasis to lung. Affect of intra-peritoneal treatment with 100 uM selenium on B16F10 experimental metastasis to lung (A) Representative photos of the lungs. Cells were injected through intra-optic venous. After 14 days mice were killed and the pulmonary nodules of B16F10 melanoma were counted. (B) Representative hematoxylin and eosin staining sections of the lungs were photographed. The arrows point to the metastatic tumors in lung. The area of the lung occupied by the colonies in the histology sections was measured microscopically. (C) The number of lung metastatic tumor.
ACKNOWLEDGEMENTS
This study was supported by the SRC/ERC program of MOST/KOSEF (grant # R11-2005-017-02002-0) and a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (grant #: 0920040).
References
1. Mancianti ML, Herlyn M. Tumor progression in melanoma: the biology of epidermal melanocytes in vitro. Carcinog Compr Surv. 1989. 11:369–386.
2. Nagao N, Nakayama T, Etoh T, Saiki I, Miwa N. Tumor invasion is inhibited by phosphorylated ascorbate via enrichment of intracellular vitamin C and decreasing of oxidative stress. J Cancer Res Clin Oncol. 2000. 126:511–518.

3. Roomi MW, Roomi N, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Inhibition of pulmonary metastasis of melanoma b16fo cells in C57BL/6 mice by a nutrient mixture consisting of ascorbic Acid, lysine, proline, arginine, and green tea extract. Exp Lung Res. 2006. 32:517–530.

4. Dua P, Ingle A, Gude RP. Suramin augments the antitumor and antimetastatic activity of pentoxifylline in B16F10 melanoma. Int J Cancer. 2007. 121:1600–1608.

5. Combs GF Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998. 79:179–192.
6. Patrick L. Selenium biochemistry and cancer: a review of the literature. Altern Med Rev. 2004. 9:239–258.
7. Han B, Wei W, Hua F, Cao T, Dong H, Yang T, Yang Y, Pan H, Xu C. Requirement for ERK activity in selenium-induced apoptosis of acute promyelocytic leukemia-derived NB4 cells. J Biochem Mol Biol. 2007. 40:196–204.

8. Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, Choi KS. Selenium induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007. 67:6314–6324.

9. Wang H, Yang X, Zhang Z, Xu H. Both calcium and ROS as common signals mediate Na(2)SeO(3)-induced apoptosis in SW480 human colonic carcinoma cells. J Inorg Biochem. 2003. 97:221–230.

10. Kramer GF, Ames BN. Mechanisms of mutagenicity and toxicity of selenium (Na2SeO3) in salmonella typhimurium. Mutat Res. 1988. 201:169–180.

11. Kim T, Jung U, Cho DY, CHung AS. Se-methylselencysteine induces apoptosis through caspase activation in HL-60 cells. carcinogenesis. 2001. 22:559–565.

12. Jung U, Zheng X, Yoon SO, Chung AS. Se-methylselenocysteine induces apoptosis mediated by reactive oxygen species in HL-60 cells. Free Radic Biol Med. 2001. 31:479–489.

13. Sherr CJ. cancer cell cycles revisited. Cancer Res. 2000. 60:3689–3695.
14. Kaeck M, Lu J, Strange R, Ip C, Ganther HE, Thompson HJ. Differential induction of growth arrest inducible genes by selenium compounds. BioChemical Pharmacology. 1997. 53:921–926.

15. Rudolf E, Rudolf K, Cervinka M. Selenium activates p53 and p38 pathways and induces caspase-independent cell death in cervical cancer cells. Cell biol Toxicol. 2008. 24:123–241.

16. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003. 36:131–149.

17. Albino AP, Juan G, Traganos F, Reinhart L, Connolly J, Rose DP, Darzynkiewicz Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: association with decreased pRb phosphorylation. Cancer Res. 2000. 60:4139–4145.
18. Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KS, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim SH, Schlessinger J, Zhang KY, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. PNAS. 2008. 105:3041–3046.

19. Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Letters. 2008. 259:82–98.

20. An WW, Wang MW, Tashiro S, Onodera S, Ikejima T. Mitogen-activated protein kinase-dependent apoptosis in norcan-tharidin-treated A375-S2 cells is proceeded by the activation of protein kinase C. Chin Med J (Engl). 2005. 118:198–203.
21. Mhaidat NM, Zhang XD, Allen J, Avery-Kiejda KA, Scott RJ, Hersey P. Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer. 2007. 97:1225–1233.

22. Kagawa S, Fujiwara T, Hizuta A, Yasuda T, Zhang WW, Roth JA, Tanaka N. p53 expression overcomes p21WAF1/CIP1-mediated G1 arrest and induces apoptosis in human cancer cells. Oncogene. 1997. 15:1903–1909.

23. Jiang C, Hu H, Malewiez B, Wang Z, Lü J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004. 3:877–884.
24. Traynor NJ, McKenzie RC, Beckett GJ, Gibbs NK. Selenomethionine inhibits ultraviolet radiation-induced p53 transactivation. Photodermatol Photoimmunol Photomed. 2006. 22:297–303.

25. Hahm E, Jin DH, Kang JS, Kim YI, Hong SW, Lee SK, Kim HN, Jung da J, Kim JE, Shin DH, Hwang YI, Kim YS, Hur DY, Yang Y, Cho D, Lee MS, Lee WJ. The Molecular Mechanisms of Vitamin C on Cell Cycle Regulation in B16F10 Murine Melanoma. J Cell Biochem. 2007. 102:1002–1010.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download