Abstract
Background
Little information is available the role of Nitric Oxide (NO) in host defenses during human tuberculosis (TB) infection. We investigated the modulating factor(s) affecting NO synthase (iNOS) induction in human macrophages.
Methods
Both iNOS mRNA and protein that regulate the growth of mycobacteria were determined using reverase transcriptase-polymerase chain reaction and western blot analysis. The upstream signaling pathways were further investigated using iNOS specific inhibitors.
Results
Here we show that combined treatment with 1,25-dihydroxyvitamin D3 (1,25-D3) and Interferon (IFN)-γ synergistically enhanced NO synthesis and iNOS expression induced by Mycobacterium tuberculosis (MTB) or by its purified protein derivatives in human monocyte-derived macrophages. Both the nuclear factor-κB and MEK1-ERK1/2 pathways were indispensable in the induction of iNOS expression, as shown in toll like receptor 2 stimulation. Further, the combined treatment with 1,25-D3 and IFN-γ was more potent than either agent alone in the inhibition of intracellular MTB growth. Notably, this enhanced effect was not explained by increased expression of cathelicidin, a known antimycobacterial effector of 1,25-D3.
Mycobacterium tuberculosis (MTB) is the greatest single infectious cause of mortality worldwide, killing roughly 2 million people per year. Estimates indicate that one-third of the world's population is infected with latent MTB. The synergy between tuberculosis (TB) and the AIDS epidemic and the surge in multidrug-resistant clinical isolates of MTB have reaffirmed TB as a primary public health threat. The development of new antituberculous agents could have a profound impact on TB therapy and on world health (1).
The high-output expression of nitric oxides (NO) in response to cytokines or to pathogen-derived molecules is an important component in host defenses against intracellular microorganisms as diverse as Toxoplasma gondii, Leishmania major, Listeria monocytogenes, Plasmodium species, Ectromelia virus, Coxsackie B3 virus, M. leprae, and MTB (2). In mice, the induction of NO and nitric oxide synthase-2 (NOS2)-derived reactive nitrogen intermediates in macrophages is a major effector mechanism for antimycobacterial action (3). There are conflicting reports as to whether human monocytes/macrophages are able to kill MTB in an iNOS-dependent manner; nevertheless, NO might play an important role in resistance to MTB infection, as seen in both experimental and human TB (4-8).
Although little research has addressed modulating factors acting on human iNOS and NO synthesis, it has been reported that the human promyelocytic cell line HL-60, when differentiated to a macrophage-like phenotype, acquires the ability to produce substantial amounts of NO upon stimulation with lipopolysaccharides (LPS) or with 1, 25-dihydroxyvitamin D3 (1,25-D3) (9). Of note, a recent study by Liu et al. (10) demonstrated that toll-like receptor (TLR) activation in human macrophages up-regulated the expression of the vitamin D receptor and vitamin D-1-hydroxylase genes, leading to the induction of the antimicrobial peptide cathelicidin, and thus killing intracellular MTB (10). Despite efforts to examine the functions of NO and inducing factors in human cells, there is no efficient method of inducing iNOS in human macrophages in vitro (11).
In the present study, we found that combined treatment with 1,25-D3 and interferon (IFN)-γ synergistically enhanced NO release and iNOS expression induced by MTB or its purified protein derivatives (PPD) in human monocyte-derived macrophages (MDMs). In addition, the activation of the nuclear factor (NF)-κB and MEK1-ERK1/2 pathways was essential for iNOS gene expression in these cells and required TLR2. Finally, this combined regimen significantly suppressed the growth of MTB in MDMs, suggesting an important role of NO in host defense against TB.
Single-cell suspension cultures of MTB H37Ra (ATCC 25177) and MTB H37Rv (kindly provided by Dr. Richard L. Friedman, University of Arizona, Tucson, AZ) were prepared as described previously (12). The tuberculin PPD used in the in vitro assays was purchased from Statens Serum Institut (Copenhagen, Denmark) and was used at a final concentration of 5.0 µg/ml.
LPS, 1,25-D3, NG-monomethyl-L-arginine (L-NMMA, a nonselective NOS inhibitor), and NG-nitro-L-arginine methyl ester (L-NAME) were purchased from Sigma (St Louis, MO). Mouse anti-human TLR2 mAb (clone TL2.1, IgG2a), mouse anti-human TLR4 mAb (clone HTA125, IgG2a), and isotype control (IC) mAb (IgG2a) were purchased from eBioscience (San Diego, CA). Specific antibodies against extracellular-regulating kinase (ERK) 1/2, phospho-(Thr202/Tyr204)-ERK1/2, p38, phospho-(Thr180/Tyr182)-p38, and phospho-IKKα/β were purchased from Cell Signaling Technology. Antibody against iNOS Ab (IgG), β-actin, IκB-α (IgG1), and MEK-1 mAb (H-8, IgG2b) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The specific inhibitors U0126 for MEK, caffeic acid phenethyl ester for NF-κB (CAPE), BAY 11-7082 for IκB-α phosphorylation, and SB203580 for p38 mitogen-activated protein kinase (MAPK) were obtained from Calbiochem (San Diego, CA). Recombinant human (rh) IFN-γ and rhtumor necrosis factor (TNF)-α were obtained from R&D systems (Minneapolis, MN). The psiRNA-h7SKGFPzeo plasmids for generating hTLR2 or hTLR4 short hairpin RNA (shRNA) were obtained from Invitrogen (Carlsbad, CA). Transfection of these plasmids into human primary MDMs was performed using amaxa Nucleofector technology (Gaithersburg, MD). Protein A-Sepharose for the MEK-1 assay was obtained from Amersham Bioscience (Freiburg, Germany), and [γ-32P]-ATP was obtained from PerkinElmer (San Jose, CA).
This study was approved by the local Ethical Committee of Konyang University Hospital's review board, which oversees studies on samples from human subjects. Healthy volunteers provided informed written consent to take part in this study. Adherent monocytes were collected from peripheral blood mononuclear cells donated by healthy subjects, as previously described (12). Human MDMs were prepared by culturing peripheral blood monocytes for 4~5 days in the presence of 0.2 ng/ml human macrophage colony-stimulating factor (Sigma). In order to show that the stimulatory capacity of mycobacteria was not the result of contamination with LPS, experiments were performed that by addition of the specific LPS-inhibiting oligopeptide polymyxin B (10 µg/ml) before mycobacterial stimulation. To assess their sensitivity to rhIFN-γ and 1,25-D3, human MDMs were incubated in the presence of these factors then stimulated with PPD or MTB.
The detection of NO was detected with minor modifications using the NO-sensitive fluorescent probe DAF-2 DA (Calbiochem) and laser-scanning confocal microscopy (model LSM 510, Zeiss), as described previously (13). NO production was also measured indirectly as nitrite accumulation in cell culture medium by using the Griess reaction (14).
Human MDMs were treated as indicated and processed for analysis by reverase transcriptase-polymerase chain reaction (RT-PCR) and western blot, as described previously (12). RT-PCR for human iNOS mRNA was performed after total RNA was extracted from the cells using TRIzol (Invitrogen). For Western blot analysis, antibodies to phosphorylated and total p38, JNK, ERK1/2, and β-actin were used at 1:1,000 dilutions. Specific bands were developed by ECL (Amersham Biosciences).
Confluent MDMs were infected with MTB in the presence or absence of pre-treatment of either IFN-γ or 1,25-D3 alone, or their combination. After incubation, the cells were lysed with ice-cold lysis buffer and in vitro MEK assay was conducted, as previously described (12). The gel was dried and autoradiography was performed to visualize the 32P-labeled MBP. Densitometry was performed on films and the fold increase was calculated as experimental sample/control sample.
For the quantification of intracellular Mtb, we employed two independent methods (15). For colony-forming units (CFUs) assay, the cells were lysed with 0.3% saponin (Sigma Chemical) to release the intracellular bacteria, after various periods of incubation. The lysates of the infected cells were sonicated in a preheated 37℃ water bath sonicator (Elma, Singen, Germany) for 5 min. Aliquots of the sonicates were then diluted tenfold in 7H9 medium. Four dilutions of each sample were plated separately on 7H10 agar plates and incubated at 37℃ with 5% CO2 for 21 days. For [3H] uracil incorporation assays, the cells were lysed then transferred to 96-well round-bottom plates and incubated in the presence of 1 µCi [3H] uracil (Amersham-Pharmacia). After 24 h, the mycobacteria were killed by treatment with paraformaldehyde (final concentration, 4%) for 30 min. [3H]uracil incorporation was measured using a beta counter (Berthold, München, Germany).
To address the role of NO during mycobacterial infection in human cells, we first characterized the pattern of in vitro NO production and iNOS expression in primary human MDMs (Fig. 1). Upon stimulation with the PPD antigen of MTB H37Ra, human primary MDMs exhibited very low levels of nitrite production as measured by Griess nitrite assays, with accumulated nitrite levels below 10 µM after at 96-h incubation (Fig. 1A). There was no cytotoxicity in cells cultured for 168 h (data not shown). Human iNOS gene induction by PPD in MDMs reached a peak at 6 h, based on RT-PCR analysis (Fig. 1B). Given that rhIFN-γ and rhTNF-α stimulate macrophages to upregulate the expression of iNOS in mice (16), the effects of these stimuli were examined on nitrite production in human MDMs. As clearly shown in Fig. 1C, the addition of IFN-γ (10 ng/ml), TNF-α (10 ng/ml), or a combination of both failed to increase the nitrite levels in human MDMs after PPD treatment. In addition, there was no significant increase in nitrite levels after combined treatment of IFN-γ and TNF-α, when compared with those cultured with media control (data not shown). These results suggest that neither IFN-γ nor TNF-α influence the up-regulation of nitrite production in human MDMs, as has been reported previously (17).
Several lines of evidence indicate that 1,25-D3 regulates host resistance to MTB. In addition to its function in the expression of the antimicrobial peptide cathelicidin (10), the vitamin D receptor triggered by 1,25-D3 is also involved in the production of substantial amounts of NO in the human promyelocytic cell line HL-60, when the cells are differentiated to a macrophage-like phenotype (9). To confirm the sensitivity of NO production to IFN-γ and 1,25-D3, we incubated human primary MDMs in the presence of these two factors. As shown in Fig. 2A, 1,25-D3 or IFN-γ alone scarcely induced an increase in nitrite production after 10 days in culture with PPD. However, the combined treatment with IFN-γ and 1,25-D3 led to a significant increase in nitrite production by human MDMs (p<0.001 vs. IFN-γ or 1,25-D3 alone on day 10 in culture with PPD; Fig. 2A). In addition, combined stimulation with IFN-γ and 1,25-D3 induced a significant and synergistic increase in NO production, as measured with the NO-sensitive fluorescent probe DAF-2 DA (p<0.001 vs. IFN-γ or 1,25-D3 alone; Fig. 2B). Furthermore, we tried to examine whether similar effects were detected in cells cultured with MTB H37Ra. As shown in Fig. 2C, combined stimulation with IFN-γ and 1,25-D3 induced a significant increase in iNOS protein expression in human MDMs treated with MTB H37Ra (Fig. 2C). The nitrite production, and expression of iNOS and NO were significantly attenuated by pretreatment with either L-NMMA or L-NAME, both of them commonly used competitive inhibitors of NO synthase (Fig. 2A~C). In all experiments, we found that the effects of combined stimulation with IFN-γ and 1,25-D3 without PPD or MTB H37Ra were negligible for induction of iNOS expression or nitrite production (data not shown). Therefore, this combined stimulation efficiently up-regulated human iNOS and NO in human macrophages.
We next investigated the molecular mechanism by which IFN-γ and 1,25-D3 induce iNOS activation. Previous studies reported that MAPKs and NF-κB regulate the expression of iNOS genes (18,19). Thus, we first tested whether incubation with IFN-γ, 1,25-D3, or both affected the activation of NF-κB in MTB H37Ra -treated human macrophages. As shown in Fig. 3A, the expression level of IκB-α was dramatically attenuated in MDMs following combined treatment for 30 min. In contrast, there was a remarkable increase in the MTB H37Ra-induced phosphorylation of IKKα/β in cells primed with IFN-γ and 1,25-D3, compared with untreated control cells or cells treated with IFN-γ or 1,25-D3 alone (Fig. 3A). Exposure to MTB H37Ra led to strong phosphorylation of ERK1/2 and p38 MAPK in human MDMs at 30 min after stimulation. Although the addition of IFN-γ or 1,25-D3 alone had no effect, their combined treatment up-regulated phospho-ERK1/2 but not phospho-p38 (Fig. 3B). In addition, a similar up-regulation of MEK1 activity was observed in MDMs primed with IFN-γ plus 1,25-D3 (Fig. 3C).
Notably, these separate responses (NF-κB, ERK1/2, and p38) were correspondingly blocked by the NOS inhibitors L-NAME and L-NMMA (Fig. 3A~C), indicating that iNOS induction activates the NF-κB and MAPK pathways in human MDMs. Further, inhibition of MEK1 with U0126 or NF-κB inhibition with specific inhibitors (CAPE and BAY11-7082) (20) reduced MTB-induced iNOS expression in human MDMs primed with IFN-γ and 1,25-D3, in a dose-dependent manner (Fig. 3D). The inhibition of p38 MAPK with SB203580 or JNK1/2 inhibition with SP600125 did not significantly diminish iNOS mRNA expression (Fig. 3D and data not shown). The results of these experiments indicate that the activation of ERK1/2 and NF-κB is necessary for the modulation of iNOS by combined IFN-γ and 1,25-D3 treatment.
It has been demonstrated that TLR2 plays an essential role in iNOS induction in vivo (21). However, there have been no reports about its role in iNOS mRNA expression in human monocytes/macrophages during MTB infection. We evaluated the roles of TLR2 and TLR4 in MTB H37Ra- or PPD-induced iNOS expression after combined treatment with IFN-γ and 1,25-D3 in human monocytes or in MDMs. In the representative experiments shown in Fig. 4A (and in data not shown), MTB H37Ra- or PPD-induced iNOS protein expression was significantly inhibited by a specific mAb against TLR2, whereas it was not affected by anti-TLR4 mAb. In addition, siRNA-mediated knockdown of TLR2, but not TLR4, resulted in diminished MTB H37Ra-induced iNOS protein expression (Fig. 4B). The knockdown of TLR2, but not TLR4, also resulted in decreased IκB-α degradation and IKKα/β phosphorylation in human MDMs (Fig. 4B). These data indicate an essential role for TLR2 in MTB H37Ra-stimulated iNOS induction by human macrophages primed with IFN-γ and 1,25-D3.
Recent studies reported that the addition of 1,25-D3 to primary human MDMs infected with virulent MTB reduced the number of viable bacilli through the induction of the antimicrobial peptide cathelicidin (10). We examined whether combined IFN-γ and 1,25-D3 treatment affected the induction of cathelicidin. As shown in Fig. 5A, PPD stimulation strongly induced the expression of cathelicidin mRNA in MDMs treated with 1,25-D3. However, the expression of cathelicidin was virtually identical between cells treated with 1,25-D3 and cells treated with 1,25-D3 plus IFN-γ (Fig. 5A). In addition, cathelicidin expression was not influenced by pretreatment with either L-NMMA or L-NAME (data not shown). These data demonstrate that the combined treatment with IFN-γ and 1,25-D3 does not act by enhancing the expression of cathelicidin.
As a physiological endpoint for gauging the importance of iNOS in antimycobacterial defenses, we quantitated intracellular mycobacterial growth using both [3H]-uracil uptake and CFU assays. To estimate the intracellular inoculum of MTB, MDMs were lysed after 1, 4, and 7 days of incubation with bacteria. Upon treatment with 1,25-D3 alone, virulent MTB H37Rv showed reduced survival in MDMs (Fig. 5B and C). In CFU assays, combined treatment with 1,25-D3 and IFN-γ resulted in a 2-fold decrease in the intracellular survival of virulent MTB H37Rv, relative to that seen with 1,25-D3 alone (Fig. 5B). The antimycobacterial effect induced by this combination was significantly attenuated by pretreatment with either L-NMMA or L-NAME (data not shown). Collectively, these results suggest that iNOS induction by the combined treatment with 1,25-D3 and IFN-γ is sufficient to influence intracellular mycobacterial growth during MTB infection.
The role of iNOS and the factor(s) affecting iNOS induction in human macrophages remain unresolved. The present data demonstrate that combined treatment with 1,25-D3 and IFN-γ synergistically enhances PPD- or MTB-induced NO release and iNOS expression in human MDMs. In mice, it has been shown that iNOS is capable of producing a large amount of NO when induced by several mediators as IFN-γ, TNF-α, LPS, and several microorganisms (22); however, under similar conditions, human macrophages produce either low levels of NO (8,23) or none at all (24,25). Recently it was shown that alveolar macrophages, but not blood monocytes, are capable of producing NO in vitro after stimulation with MTB and its secretory antigens (26). Regarding NO induction, our findings are consistent with previously published data showing that iNOS expression in monocytes/macrophages in humans does not appear to be up-regulated by IFN-γ and TNF-α (17). Although stimulation with rhIFN-γ and rhTNF-α (either separately or together) failed to increase the level of NO, combined treatment with IFN-γ and 1,25-D3 synergistically stimulated the production of NO and iNOS activation in human MDMs. Combined 1,25-D3 and IFN-γ treatment may represent a physiological cell culture system for the induction of iNOS in human macrophages derived from the monocytes of healthy donors. Thus, our work establishes an ideal in vitro model environment for modulating the NO-dependent antimycobacterial defenses of human MDMs.
Previous studies have suggested that a NO-independent pathway is responsible for the direct antimicrobial activity of human monocytes/macrophages against intracellular bacteria via TLRs (27); however, this conclusion was based on in vitro findings that may differ critically from those with differentiated macrophages in vivo and ex vivo (11). Our results suggest that NO plays an indispensable role in human defenses against mycobacterial infections because the combined regimen, which was significantly modulated by iNOS inhibitors, significantly suppressed the growth of MTB (see Fig. 5B and C; data not shown). MTB-infected human alveolar macrophages are capable of producing NO, which is correlated with intracellular growth inhibition of mycobacteria in alveolar macrophages (8). Additionally, when macrophages expressing iNOS were recovered from patients and infected with mycobacteria in vitro, iNOS inhibitors abolished the antimycobacterial activity induced by the macrophages (28). Moreover, it was previously demonstrated that stimulation of monocytes/ex vivo-matured macrophages from active TB patients with proinflammatory cytokines leads to NO production and lowers the CFU of MTB in these cells (29). Taken together, NO may serve directly or indirectly as a mycobactericidal mediator in human monocytes/macrophages.
Our data indicate that ERK-1/2 and NF-κB activation is necessary for the modulation of iNOS by combined IFN-γ and 1,25-D3 treatment. Specific inhibition of either NF-κB or the ERK-1/2 pathway revealed the specific role of the NF-κB and ERK-1/2 pathways in IFN-γ-inducible NOS expression and NO production in human macrophages (30). In addition, ERK-1/2 activation results in Signal transducer and activator of transcription 1α (STAT1α) phosphorylation in IFN-γ-stimulated macrophages, and both ERK-1/2 and STAT1α are key players in the IFN-γ-inducible generation of NO by macrophages (31). Furthermore, our data indicate that combined treatment with IFN-γ and 1,25-D3 leads to significant MEK1 activation and IKKα/β phosphorylation, upstream of ERK-1/2 and IκBα, respectively. In addition, robust activation of MAPK and NF-κB is also dependent on iNOS expression in human macrophages, as these signaling pathways are also modulated by intracellular NOS inhibitors. Thus, our data also indicate a role of NO in mediating the regulation of key transcription factors targeted by cellular signaling, as suggested by previous studies (32).
This is the first study to demonstrate an indispensable role for TLR2 in MTB-stimulated iNOS induction by human macrophages primed with IFN-γ and 1,25-D3. Our results confirm previous findings regarding vitamin D as a key link between TLR activation and antibacterial responses in innate immunity (10). Vitamin D3 is known to possess a variety of immunomodulatory properties, including effects on both myeloid and lymphoid cells (33), and several lines of evidence indicate that 1,25-D3 regulates host resistance to MTB. Recently, it was shown that priming monocytic cells with 1,25-D3 strongly influences their sensitivity to LPS and different TLR2 triggers [lipoteichoic acid (LTA), trispalmitoyl-cysteyl-seryl-lysyl-lysyl-lysyl-lysine (Pam3Cys) and peptidoglycan (PGN)] (34). Moreover, 1,25-D3 deficiency and vitamin D receptor polymorphisms have been linked to increased susceptibility to MTB and Mycobacterium leprae (35-37), and in murine studies, 1,25-D3 has been shown to activate the antimycobacterial activities of mononuclear phagocytes (38,39).
Furthermore, a direct correlation exists between the levels of IFN-γ and 1,25-D3 in the pleural spaces, which suggests that IFN-γ is an important peripleural regulator of macrophage 1,25-D3 synthesis (40). Moreover, exposure of primary monocytes, alveolar macrophages, or monocytic THP-1 cells to IFN-γ markedly enhances macrophage 1,25-D3 production (41), suggesting that cross-talk occurs between the cytokine and 1,25-D3. Thus, our data provide additional strong evidence that the vitamin D-mediated antimicrobial mechanism in human macrophages is likely augmented by T cell-dependent adaptive immune mechanisms, including signals for macrophage activation by cytokines, especially IFN-γ.
It is presently unclear why combined treatment with 1,25-D3 and IFN-γ did not produce a synergistic effect on nitrite production in 96-h cultures, as measured by the Griess method, given its effects on NO production and iNOS expression (Fig. 2A~C). As IFN-γ and 1,25-D3 show potential superoxide anion-generating activity upon the stimulation of human monocytic cells (42), we asked whether stimulation with 1,25-D3, IFN-γ, or both could elicit the generation of superoxide in human MDMs. We found that IFN-γ significantly enhanced superoxide anion generation in 1,25-D3-treated MDMs (data not shown). NO is a short-lived diatomic free-radical species synthesized by NOS that reacts with superoxide anion to form reactive peroxinitrite (OONO-) (43). Therefore, the antimycobacterial activities induced by the combination of 1,25-D3 and IFN-γ may also be attributable to the generation of a potent oxidant, such as peroxinitrite, by superoxide and NO. Further studies are necessary to reveal the precise mechanisms by which the combined regimen activates antimycobacterial activities related to the generation of reactive oxygen and nitrogen species.
Figures and Tables
 | Figure 1Nitrite production and iNOS mRNA expression by PPD in human monocyte-derived macrophages (MDMs). MDMs were pretreated with TNF-α (10 ng/ml), or IFN-γ (10 ng/ml), alone or in combination, for 18 h. The following pretreatment, PPD (5 µg/ml) was added to all cultures. (A) Kinetics of PPD-induced nitrite production. (B) Analysis of iNOS mRNA expression after PPD treatment. Upper panel, Representative gel from three independent experiments with similar results is shown. Lower panel, Densitometric assessment of three independent replicates (for each experiment) with similar results. (C) Effect of IFN-γ, TNF-α, or both on PPD-induced nitrite production at 96 h. Results are representative of three experiments. *p<0.05, **p<0.01 vs. untreated control. |
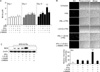 | Figure 21,25-D3 synergizes with IFN-γ to increase NO, nitrite, and iNOS protein levels in human monocyte-derived macrophages (MDMs). MDMs were pretreated with IFN-γ, or 1,25-D3 (20 nM), alone or in combination, for 18 h, with or without L-NMMA (100 µM) or L-NAME (100 µM). Following pretreatment, PPD or MTB H37Ra (MOI = 1) was added to all cultures. (A) Effect of IFN-γ, 1,25-D3, or both on PPD-induced nitrite production over time (at 1, 4, and 10 days). (B) PPD-induced NO generation in human MDMs pretreated with IFN-γ, 1,25-D3, or both. The generation of NO was detected by staining with DAF-2 DA (10 µM) for 1 h and monitored by confocal microscopy. Upper panel, representative immunofluorescence. Lower panel, quantitative analysis (C) Expression of iNOS protein by MTB H37Ra-stimulated MDMs pretreated with IFN-γ, 1,25-D3, or both at 6 h. Western blot analysis was performed using rabbit anti-iNOS Ab. Results are representative of three experiments. ***p<0.001 vs. PPD-treated control. |
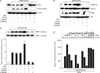 | Figure 3iNOS expression induced by IFN-γ and 1,25-D3 involves NF-κB or the ERK1/2 pathway. (A~C) MDMs were pre-incubated with IFN-γ, 1,25-D3, or both for 18 h with or without L-NMMA or L-NAME. Cells were then infected with MTB H37Ra (MOI=1) for 30 min. (A, B) Western blot analysis was performed for IκB-α, p-IKKα/IKKβ, p-ERK1/2, and p-p38. (C) MEK1 assay. Upper panel, Representative gel from three independent experiments with similar results is shown. Lower panel, Densitometric assessment of three independent replicates (for each experiment) with similar results. (D) RT-PCR analysis for iNOS mRNA. Human MDMs were infected with MTB H37Ra (MOI=1) for 6 h in the absence or presence of different protein kinase inhibitors [U0126 (5, 10, 20µM), SB203580 (1, 5, 10µM), CAPE (0.1, 1, 10µM), or BAY 11-7082 (1, 5, 10µM) for 45 min]. The β-actin mRNA level was used as a control. Upper panel, Representative gel from three independent experiments with similar results is shown. Lower panel, Densitometric assessment of four independent replicates (for each experiment) with similar results. ***p<0.001 vs. untreated control. |
 | Figure 4Induced iNOS expression is dependent on TLR2 but not TLR4. (A) MDMs were pre-incubated with anti-TLR2, anti-TLR4, or isotype-control mAbs (10 µg/ml), followed by treatment with IFN-γ, 1,25-D3, or both for 18 h. Cells were then infected with MTB H37Ra (MOI=1) for 18 h. Western blot analysis was performed using rabbit anti-iNOS Ab. (B) Effects of specific hTLR2 or hTLR4 gene silencing on MTB H37Ra -stimulated iNOS expression of human MDMs pretreated with IFN-γ plus 1,25-D3. Gene silencing using shRNAs generated by psiRNA-h7SKGFPzeo plasmids was performed by transient transfection using amaxa Nucleofector technology. At 18 h after transfection, the cells were treated with IFN-γ plus 1,25-D3 for 18 h, followed by stimulation with MTB H37Ra (MOI=1) for 30 min. Cell lysates were prepared and analyzed on Western blots for the expression of iNOS, TLR2, TLR4, IkB-α, and p-IKKα/IKKβ. The β-actin level was used as a control. Results are representative of three experiments. |
 | Figure 5IFN-γ plus 1,25-D3 induces antimycobacterial activity against MTB. (A) Densitometric analysis for cathelicidin (LL-37) mRNA expression in human MDMs stimulated by PPD after an 18-h pretreatment with IFN-γ, 1,25-D3, or both. Cathelicidin levels are expressed relative to β-actin levels. (B, C) Antimicrobial activity by human MDMs pretreated with IFN-γ, 1,25 D3, or both. Cells were pre-incubated for 18 h and infected with MTB H37Rv (MOI=1). At day 7, the number of viable bacteria was assessed by CFU assay. Results are representative of seven experiments. (C) At the time points shown, the cells were lysed, and [3H]-uracil incorporation into the released bacteria was measured. Results are representative of six experiments. *p<0.05, **p<0.01, ***p<0.001 vs. MTB H37Rv-treated control. |
ACKNOWLEDGEMENTS
This work was supported by the Korea Science & Engineering Foundation through the Infection Signaling Network Research Center (R13-2007-020-01000-0) at Chungnam National University. The authors have no financial conflict of interests. We thank R. L. Friedman for M. tuberculosis H37Rv.
References
1. Tomioka H. Prospects for development of new antimycobacterial drugs. J Infect Chemother. 2000. 6:8–20.

2. Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992. 175:1111–1122.

3. Flynn JL, Scanga CA, Tanaka KE, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998. 160:1796–1803.
4. Lon R, Light B, Talbot JA. Mycobacteriocidal action of exogenous nitric oxide. Antimicrob Agents Chemother. 1999. 43:403–405.

5. Yu K, Mitchell C, Xing Y, Magliozzo RS, Bloom BR, Chan J. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber Lung Dis. 1999. 79:191–198.

6. Nicholson S, Bonecini-Almeida Mad G, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JR. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996. 183:2293–2302.

7. Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998. 11:809–815.

8. Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber Lung Dis. 1997. 78:247–255.

9. Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998. 66:5314–5321.

10. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006. 311:1770–1773.

12. Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, Akira S, Paik TH, Jo EK. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 2007. 9:382–389.

13. Madrigal JL, Russo CD, Gavrilyuk V, Feinstein DL. Effects of noradrenaline on neuronal NOS2 expression and viability. Antioxid Redox Signal. 2006. 8:885–892.

14. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1998. 141:2407–2412.
15. Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009. 182:3696–3705.

16. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997. 15:323–350.

17. Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993. 167:1358–1363.

18. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998. 16:225–260.

19. Jaramillo M, Gowda DC, Radzioch D, Olivier M. Hemozoin increases IFN-gamma-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase- and NF-kappa B-dependent pathways. J Immunol. 2003. 171:4243–4253.

20. Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappaB. Proc Natl Acad Sci USA. 1996. 93:9090–9095.

21. Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003. 47:327–336.

22. Chan ED, Winston BW, Uh ST, Wynes MW, Rose DM, Riches DW. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-γ and TNF-α in mouse macrophages. J Immunol. 1999. 162:415–422.
23. Denis M. Tumor necrosis factor and granulocyte macrophage colony stimulating factor stimulate human macrophage to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991. 9:380–387.
24. Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, Haney AF, Granger DL. Human mononuclear phagocytes inducible nitric oxide synthase (NOS): analysis if iNOS mRNA, iNOS protein, bioprotein and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995. 86:1184–1195.

25. Padgett EL, Pruett SB. Evaluation of nitrite production by human monocyte derived macrophages. Biochem Biophys Res Commun. 1992. 186:775–781.
26. Sable SB, Goyal D, Verma I, Behera D, Khuller GK. Lung and blood mononuclear cell responses of TB patients to mycobacterial proteins. Eur Respir J. 2007. 29:337–346.

27. Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001. 291:1544–1547.

28. Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997. 65:3644–3647.

29. Bose M, Farnia P, Sharma S, Chattopadhya D, Saha K. Nitric oxide dependent killing of Mycobacterium tuberculosis by human mononuclear phagocytes from patients with active tuberculosis. Int J Immunopathol Pharmacol. 1999. 12:69–79.
30. Jaramillo M, Naccache PH, Olivier M. Monosodium urate crystals synergize with IFN-gamma to generate macrophage nitric oxide: involvement of extracellular signal-regulated kinase 1/2 and NF-kappa B. J Immunol. 2004. 172:5734–5742.

31. Blanchette J, Jaramillo M, Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003. 108:513–522.

32. Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002. 14:879–897.

33. Manolagas SC, Hustmyer FG, Yu XP. Immunomodulating properties of 1,25-dihydroxyvitamin D3. Kidney Int Suppl. 1990. 29:S9–S16.
34. Remer KA, Brcic M, Sauter KS, Jungi TW. Human monocytoid cells as a model to study Toll-like receptor-mediated activation. J Immunol Methods. 2006. 313:1–10.

35. Davies PD. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985. 66:301–306.

36. Roy S, Frosham A, Saha B, Hazra SK, Mascie-Taylor CG, Hill AV. Association of vitamin D receptor genotype with leprosy type. J Infect Dis. 1999. 179:187–191.

37. Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000. 355:618–621.

38. Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987. 55:2945–2950.

39. Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986. 57:159–163.
40. Adams JS, Modlin RL, Diz MM, Barnes PF. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab. 1989. 69:457–460.

41. Dusso AS, Kamimura S, Gallieni M, Zhong M, Negrea L, Shapiro S, Slatopolsky E. Gamma-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997. 82:2222–2232.

42. Kikuchi H, Iizuka R, Sugiyama S, Gon G, Mori H, Arai M, Mizumoto K, Imajoh-Ohmi S. Monocytic differentiation modulates apoptotic response to cytotoxic anti-Fas antibody and tumor necrosis factor alpha in human monoblast U937 cells. J Leukoc Biol. 1996. 60:778–783.

43. Jourd'heuil D, Mills L, Miles AM, Grisham MB. Effect of nitric oxide on hemoprotein-catalyzed oxidative reactions. Nitric Oxide. 1998. 2:37–44.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download