Abstract
Background
The present study examines a hypothesis that short allergen-derived peptides may shift an Aspergillus fumigatus (Afu-) specific TH2 response towards a protective TH1. Five overlapping peptides (P1-P5) derived from Asp f1, a major allergen/antigen of Afu, were evaluated for prophylactic or therapeutic efficacy in BALB/c mice.
Methods
To evaluate the prophylactic efficacy, peptides were intranasally administered to naïve mice and challenged with Afu-allergens/antigens. For evaluation of therapeutic efficacy, the mice were sensitized with Afu-allergens/antigens followed by intranasal administration of peptides. The groups were compared for the levels of Afu-specific antibodies in sera and splenic cytokines evaluated by ELISA. Eosinophil peroxidase activity was examined in the lung cell suspensions and lung inflammation was assessed by histopathogy.
Results
Peptides P1-, P2- and P3 decreased Afu-specific IgE (84.5~98.9%) and IgG antibodies (45.7~71.6%) in comparison with Afu-sensitized mice prophylactically. P1- and P2-treated ABPA mice showed decline in Afu-specific IgE (76.4~88%) and IgG antibodies (15~54%). Increased IgG2a/IgG1 and IFN-γ/IL-4 ratios were observed. P1-P3 prophylactically and P1 therapeutically decreased IL-5 levels and eosinophil peroxidase activity. P1 decreased inflammatory cells' infiltration in lung tissue comparable to non-challenged control.
Allergic bronchopulmonary aspergillosis (ABPA), an allergic disorder induced by the fungal pathogen Aspergillus fumigatus (Afu), is characterized by the presence of both type I and type III mediated hypersensitivity reactions leading to increased levels of total IgE, specific IgE (Afu-IgE), specific IgG (Afu-IgG), blood and pulmonary eosinophilia and upregulation of TH2 pathway with the secretion of IL-4, IL-5, IL-6, IL-10 and IL-13 cytokines (1). Increased incidences of ABPA in patients of cystic fibrosis (1~15%), asthma (1~11%) and Aspergillus skin test-positive asthmatic subjects (24~28%) demand therapeutics that can potentially inhibit pathological conditions induced by Afu (2-4). Importance of TH2 cytokine biology in propagating inflammatory and pathological responses in ABPA, suggest that inhibition of TH2 response could provide promising therapy against ABPA. It is well established that TH1 cells produce cytokines which downregulate TH2-induced allergic responses (5). In view of the above fact, restoring TH1/TH2 balance by inducing allergen-specific TH1 responses has been found to be a promising approach in allergen-specific immunotherapy (SIT). SIT shifts the balance of T-cell subsets from TH2- to TH1-type to produce a long-term, antigen-specific, protective immune response with an increase in allergen-specific "blocking" IgG and induction of IL-10 secreting T-regulatory or suppressor cells (5-11). However, use of allergen-SIT has been cautioned in fungal allergic diseases as type III reactions are contributors to pathogenesis rather than protection. There are no reports for allergen-SIT for ABPA but has been reported for asthma induced by Cladosporium herbarum and Alternaria alternata using purified allergen extracts (12-14). Due to risks involved with whole allergen-immunotherapy and benefits of a standardized manufacture of peptides, attention has been focused to immunotherapy with peptides that display reduced IgE-binding while retaining T-cell epitopes. It is now plausible to attempt the peptide-specific therapy against Afu-induced allergic diseases owing to availability of annotated genome sequence of Afu and knowledge of characterized immunodominant allergens/antigens. Relevant epitopes in these allergens/antigens have been identified both by prediction algorithms and by experiment (15-22).
One of the earliest known and characterized allergen/antigen of Afu is Asp f1 with 85% of allergic aspergillosis patients showing IgE antibodies to Asp f1 (17,18,23-25). Asp f1 is a cytotoxic ribonuclease hindering its use for immunotherapy in a native form owing to the possible toxicities and IgE-mediated side effects. The present study was undertaken to identify promising Asp f1 peptide candidates with capability of programming the functional activity of Asp f1-specific T cells to TH1 type. Studies by various groups suggested presence of T cell epitopes in the N-terminal region of Asp f1 (1 to 20 amino acid residues) (15,16,26). In an earlier study, we evaluated five overlapping peptides (P1-P5) from the N-terminal region of Asp f1 for diagnostic relevance (19). The same peptides in the present study were predicted for their binding to MHC class II alleles of BALB/c mice, and evaluated for induced cytokine profile in splenocytes of Afu sensitized mice (referred as ABPA mice). Three of these peptides (P1-P3) with significant stimulation index were prophylactically administered to naïve mice, and mice were evaluated for protection against challenge with Afu allergens/antigens (Fig. 1). Studies were also conducted to investigate whether these peptides can therapeutically downregulate established TH2 responses in murine model of pulmonary fungal hypersensitivity caused by Afu (which mimic immunological profile of ABPA).
As reported earlier, five overlapping synthetic peptides P1-P5 from the N-terminal region of Asp f1 were observed to be immunodominant (19). Binding affinity (IC50) of these peptides to major human HLA-DR molecules was predicted using Support Vector Machine (SVM) method (27). The binding with BALB/c MHC class II molecules i.e., I-Ad and I-Ed was predicted by the available online server, PREDBALB/C (28).
For SVM prediction method, the dataset of peptides with pre-determined IC50 values with HLA-DR2, DR4, DR5, DR7 and preponderant alleles like DRB1*1501 (DR2) and DRB1*0401 (DR4) were collected from AntiJen database (http://www.jenner.ac.uk/antijen/) and MHCBN database (http://www.imtech.res.in/raghava/mhcbn). This dataset containing the peptides and their binding affinity (experimentally determined) was used as an input for the Support Vector Machine. SVM parameters were optimized in order to develop the best model and the method was validated using five-folds cross validation and leave one out cross validation. Correlation between the actual and the predicted affinity values was calculated and the model at which the correlation was the highest (>0.5) was selected as the SVM model for prediction of binding affinities of peptides P1-P5 (27,29). Peptides were arbitrarily classified based on their predicted IC50 values (high-affinity binders: IC50≤500 nM, medium-affinity binders: 500 nM<IC50≤1,500 nM, low-affinity binders: 1,500<IC50≤5,000 nM and non-binders: 5,000<IC50).
As reported earlier, peptides P1-P5 were synthesized using solid phase methodology by standard Fmoc-chemistry and purified by reverse-phase HPLC on an analytical CE-18 column (Applied BioSystems) and characterized by FAB-MS (Fast atom bombardment mass spectrophotometry) (Jeol JMS-360) (19). Amino acid sequences of the peptides are given in the Table I.
Three-week culture filtrate allergens/antigens (3wcf; protein enriched antigenic fraction) of Afu (clinical strain 285, isolated from the sputum of an ABPA patient visiting the V. P. Chest Institute, Delhi, India) used for the following experiments were prepared as described earlier (19,30). The three-week culture filtrate was observed to be enriched with diagnostically relevant allergens including Asp f1 as reported by our group and others (18,23). The suitability of the antigen was determined by its immunoreactivity with specific IgG and IgE antibodies in sera of clinically confirmed ABPA patients by ELISA and Western blotting techniques.
Specific-pathogen-free, 6~8 week old BALB/c mice were obtained from the National Institute of Nutrition, Hyderabad, India. They received Purina chow and acidified water ad libitum. Mice were randomized before experiments were performed. All procedures involving the handling of mice were approved by the institute's animal ethics committee.
A murine model of pulmonary hypersensitivity was prepared as described previously (30) and called "ABPA mice" for descriptive convenience.
To assess whether Asp f1 peptides (P1-P5) can stimulate Afu-sensitized splenocytes of ABPA mice, in vitro splenocytes proliferation was carried out as described previously (19,34). Briefly, mice were sacrificed and spleens were removed aseptically and placed in an ice-cold PBS. After gentle dissociation, cellular suspensions were cultured in a volume of 100 µl in 96-well flat-bottom plates at the density of 2×105 cells. Peptides and 3wcf (final concentration -5 µg/ml) were added into the culture medium and allowed to proliferate in RPMI-1640 medium with 10% (v/v) fetal calf serum and 10 µg/ml gentamicin at 37℃ and 5% CO2 for 72 hr. 10 µl of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma Aldrich, Delhi, India) (5 mg/ml) was put in wells, kept for 2~3 hr; cells were lysed with 0.04 N isopropanol-HCl and read at 572 nm.
Stimulation Index was obtained as: OD572 nm value after stimulation with 3wcf or peptides/OD572 nm value without stimulation.
The immunization/treatment schedule followed for prophylactic and therapeutic regimens was optimized considering previous studies (31,32). Schematic details of the schedule are outlined in Fig. 1. The mice were divided into five groups for each regimen (prophylactic and therapeutic), with five mice in each group: Non-challenged control mice, P1-treated mice, P2-treated mice, P3-treated mice and PBS-treated ABPA or Afu challenged mice.
Splenocytes of various groups of mice i.e., peptide-treated, PBS-treated and control mice after both prophylactic and therapeutic regimens, were allowed to proliferate as described in previous section. Peptides and 3wcf (final concentration -5 µg/ml) were added into the culture medium and allowed to proliferate in RPMI-1640 medium (Sigma Aldrich, Delhi, India) with 10% (v/v) fetal calf serum (Biological Industries, Israel) and 10 µg/ml gentamicin at 37℃ and 5% CO2 for 72 hr. Splenic supernatants obtained were assayed for IL-4, IFN-γ and IL-5 levels according to the manufacturer's instructions (BD OptEIA kits, BD Pharmingen, CA, USA).
The Afu-IgG and Afu-IgE levels in the serum were measured by ELISA, as reported previously (33). The serum dilutions used for IgG and IgE estimation were 1:100 (v/v) and 1:10 (v/v), respectively. Horseradish peroxidase labeled-goat anti-mouse IgG (Sigma Aldrich, Delhi, India) and IgE (Bethyl Labs, TX, USA) conjugates were used at 1:1,000 (v/v) dilutions. The optical densities at 490 nm were measured with an ELISA reader (SpectraMAX 190, Molecular Devices, CA, USA).
The Afu-IgG1 and IgG2a responses were measured by ELISA as described earlier (33). The serum dilution used for IgG1 and IgG2a estimation was 1:100 (v/v). Horseradish peroxidase-labeled rat anti-mouse IgG1 and IgG2a (BD pharmingen, CA, USA) antibodies were used at 1:1,000 (v/v) dilutions. The optical densities at 490 nm were measured with an ELISA reader (SpectraMAX 190, Molecular Devices, CA, USA).
EPO assay, an indicator of number of eosinophils in activated state, was performed as described earlier (30). Briefly, lung cell suspension (200 µl/well) was plated in a 96-well tissue culture plate and incubated in a humidified CO2 incubator at 37℃ for 48 hours. The medium was aspirated and o-phenylene diamine (OPD) was added (100 µl of 1 mM solution was prepared using sterile PBS containing 0.1% vol/vol Triton X-100 and 0.0125% vol/vol H2O2). After 30-minute incubation at room temperature, the color reaction was terminated by addition of 50 µl of 4 N H2SO4, and the A492 was measured.
Lungs removed from the sacrificed mice were trimmed of extraneous tissue and fixed in 10% (v/v) formaldehyde and stored at 4℃. The tissue sections, made using a microtome and stained with hematoxylin and eosin, were examined at x20.
Unless stated otherwise, all data are expressed as mean±standard deviation (SD), with five mice per group. Differences between the mean values of untreated and treated groups were analyzed using student's unpaired t-test available at the site http://home.clara.net/sisa/t-test/htm. A p-value <0.05 was considered statistically significant. The data reported was pooled from three independent experiments, unless specified otherwise.
Peptides P1-P5 were observed to be predicted binders using PREDBALB/c server to MHC class II alleles, IAd and IEd (data not shown). Peptides P1-P5 exhibited medium binding affinity (500 nM<IC50≤1,500 nM) to multiple HLA-DR alleles such as HLA-DR2, HLA-DR4, HLA-DR5, HLA-DR7 and also with preponderant alleles such as HLADRB1*1501 (DR2) and HLADRB1*0401 (DR4) following SVM method except P1 and P5 which showed low binding to DRB1*1501 (Table II). With alleles DR2, DR4 and DR5, binding affinities were predicted to be higher for peptides P1 and P3. Peptides P2 showed higher binding to DR2 and DR4 allele while P5 peptide with alleles DR4 and DR5. Peptide P1 showed the highest binding (IC50~90 nM) with DR5 allele. Peptide P2 also showed higher binding to preponderant alleles DRB1*1501 and DRB1*0401 when compared to other peptides (Table II).
ABPA mice splenocytes were challenged in vitro with 3wcf and peptides P1-P5 (5 µg/ml) and were analyzed for their stimulation index. Peptides P1-P3 showed higher stimulation index (up to 2.4-fold) indicating that they are Asp f1-derived immunodominant epitopic regions recognized by Afu-sensitized splenocytes (data not shown). Peptides, P1-P3 were therefore selected for further evaluation in BALB/c mice.
A significant increase in Afu-IgE and Afu-IgG levels was observed in the groups of ABPA mice before any treatment and in Afu-challenged mice immunized for two weeks with 3wcf (without any prior treatment), in comparison with those of non-challenged control mice immunized with PBS alone (p<0.05) (Fig. 2).
In prophylactic regimen, P1-, P2- and P3-treated naïve mice showed significant suppression in Afu-specific IgE levels i.e., 98.9%, 94% and 84.5%, respectively on day 36 (Fig. 2A, p <0.05) in comparison to PBS-treated Afu-challenged mice. Similarly, when compared to PBS-treated Afu-challenged mice, there was significant suppression in Afu-specific IgG levels up to 71.6%, 69.4% and 45.7% in P1-, P2- and P3-treated mice, respectively after two-week challenge of Afu allergen (Fig. 2A, p<0.05).
When compared to PBS-treated ABPA mice, therapeutic administration of peptides P1 and P2 for 3 consecutive days resulted in decrease of Afu-IgE levels up to 76.4% and 88%, respectively on day 42 of the study (Fig. 2B, p<0.05). However, P3-treated ABPA mice did not show significant decrease (~12.7%) in specific IgE levels (Fig. 2B, p>0.05).
In comparison to PBS-treated ABPA mice, P1- and P3-treated ABPA mice showed 53.6% and 39.8% decrease in Afu-specific IgG levels following therapeutic regimen, respectively (Fig. 2B, p<0.05). No significant decrease in Afu-specific IgG levels was observed in P2-treated ABPA mice following therapeutic regimen (Fig. 2B, p>0.05).
P1-, P2- and P3-treated naïve mice in a prophylactic regimen showed decreased Afu-IgG1 levels while specific IgG2a levels remained unaltered after challenged to Afu allergens for two weeks (Fig. 3A, p<0.05). The IgG2a/IgG1 ratios were 4-fold, 13.4-fold and 9.4-fold higher than PBS-treated Afu-challenged mice in P1-, P2- and P3-treated mice, respectively. In therapeutic approach, IgG2a/IgG1 ratios were 3-fold and 7.15-fold higher than PBS-treated ABPA mice in P1- and P2-treated ABPA mice, respectively (Fig. 3B, p<0.05). P3-treated ABPA mice showed unaltered levels of specific IgG1 antibodies with decreased IgG2a levels thus lowering the ratio of IgG2a/IgG1 in comparison to PBS-treated ABPA mice (Fig. 3B, p>0.05).
Ratios of IFN-γ/IL-4 in splenic supernatants of PBS-treated Afu-challenged and ABPA mice were found to be 1.98±0.049 and 1.38±0.069, respectively (Fig. 4A).
In prophylactic regimen, P1-, P2 and P3-treated naïve mice showed 9.74-fold, 12.4-fold and 3.8-fold higher IFN-γ/IL-4 ratios, when compared to PBS-treated Afu-challenged mice (Fig. 4A, p<0.05). While in a therapeutic regimen, P1-, P2- and P3-treated mice showed 9.2-fold, 16.2-fold and 1.9-fold higher IFN-γ/IL-4 ratios, when compared to PBS-treated ABPA mice (Fig. 4B, p<0.05).
In prophylactic regimen, P1-, P2- and P3-treated naïve mice showed marked reduction of 25%, 47.2% and 46.2% in lung EPO activity, respectively in comparison to PBS-treated Afu-challenged mice (Fig. 5A, p<0.05). Therapeutic approach, when compared to PBS-treated ABPA mice, P1-treated ABPA mice showed up to 30.8% decrease in lung eosinophilic index (EPO) (Fig. 5A, p<0.05). P2- and P3-treated ABPA mice showed unaltered levels of lung EPO index (Fig. 5A, p>0.05).
The reduction in the lung EPO activity correlated well with the concomitant reduction in the IL-5 production (Fig. 5B). Following the prophylactic regimen, when compared with PBS-treated Afu-challenged mice, levels of IL-5 cytokine were found to be significantly inhibited in P1 (37.7%), P2 (71.6%) and P3 (61.9%)-treated naïve mice (Fig. 5B, p<0.05). In therapeutic regimen, IL-5 levels were significantly decreased in P1-treated ABPA mice (up to 26.5%) (Fig. 5B, p<0.05). P2- and P3-treated ABPA mice showed unaltered levels of IL-5 cytokine (Fig. 5B, p>0.05).
As shown in Fig. 6, histopathological examination of lung sections of Afu-challenged and ABPA mice treated with PBS alone revealed the extensive chronic inflammatory infiltrates, mainly representing lymphocytes and eosinophils (Fig. 6A, B). These inflammatory cells were frequently located around perivascular and peribronchiolar areas. Mice in the PBS-treated non-challenged control groups had normal bronchi and parenchyma and had no conspicuous cellular infiltrates (Fig. 6C, D). When examined on 10th day of prophylactic (day 36), the infiltration was drastically reduced in P1-treated mice comparable to that of PBS-treated control mice (Fig. 6E). P2-treated mice also exhibited decrease in lung tissue infiltration when compared to PBS-treated Afu challenged mice (Fig. 6F). No marked reduction in tissue infiltration was observed in P3-treated mice when compared to PBS-treated Afu-challenged mice (Fig. 6G). In therapeutic (day 42) regimen, the infiltration was markedly reduced in P1 comparable to PBS-treated non-challenged control mice (Fig. 6H). P2 and P3-treated ABPA mice showed decrease in infiltration when compared to PBS treated ABPA mice (Fig. 6I, J).
The results demonstrate that peptides derived from the immunodominant N-terminal region of Asp f1, a major allergen/antigen of Afu, have immunomodulatory properties leading to predominant allergen-specific TH1 responses. Several studies have reported immunodominant T-cell epitopes derived from house dust mite (Der p1), cat allergens (Fel d1), dog allergen (Can f1), and bee venom allergen (PLA-2) which inhibited allergen-specific TH2 responses by skewing the immune response towards TH1 (31,32,35-38). Few of these studies also emphasized that administration of single immunodominant peptide results in inhibition of responses to the entire allergen, indicating that not all allergen-derived T-cell epitopes have to be present to inhibit allergic responses (32,36). In contrast to other IgE-mediated allergies, immunotherapeutic interventions against mold allergy have been challenging due to multiple allergenic components and difficulty in standardization of fungal extracts (39-41). Owing to the identification of major allergens/antigens of Afu and their immunodominant T-cell epitopes, safer peptide based-immunotherapy could be more promising approach against Afu -induced allergies (16,19,26).
Evaluation of Asp f1 peptides (P1-P3) in H2Id BALB/c mice, high responders to Afu allergen, indicated capability of these peptides in redirecting cytokine profile from TH2 towards TH1 in both naïve and ABPA mice. Significant suppression and decline in Afu-specific IgE levels observed in prophylactic (in P1-, P2- and P3-treated naïve mice) and therapeutic (in P1- and P2-treated ABPA mice) regimen, respectively, were associated with increased IFN-γ/IL-4 ratios. Subsequent increase in IgG2a/IgG1 ratios observed in the similar groups of mice and suppressed Afu-specific IgG levels markedly in prophylactic (in P1-, P2- and P3-treated) regimen, indicated the shift from predominant TH2 to TH1 type. It has been reported that in the murine system IFN-γ regulates the isotypes of antibodies secreted during both in vivo and in vitro humoral responses, leading to the stimulation of IgG2a secretion and in the suppression of secretion of both IgG1 and IgE antibodies (42). Kozutsumi et al studied the effect of Japanese cedar pollinosis Cry-consensus peptide (CCP) on Cry j1-specific TH1/TH2 responses using B10.S as a mice model. Corroborating with our results, CCP-treated (subcutaneously) mice exhibited increase in Cry j1-specific IgG2a antibodies, decreased IgG1 and IgE production, and increased secretion of IFN-γ cytokine by splenocytes (43). Similarly, treatment of PLA2 (a major bee venom allergen)-sensitized mice with intraperitoneal injections of a mixture of three overlapping peptides (44~60 mer) spanning the entire PLA2 molecule (100 µg/peptide) induced a sharp drop in PLA2-specific IgE antibodies, an increase in specific IgG2a antibodies with T cell cytokine secretion shifting from a TH2 to TH1 profile (44). In a separate study, it has been reported that peptides-derived from PLA-2 were able to modulate an established TH2 response, indicated by decreased levels of PLA2-specific IgE and IgG1 antibodies, and declined IL-4/IFN-γ ratio (31).
Successful immunotherapy has also been shown to be associated with reductions in numbers of eosinophils at sites of inflammation (45,46). The recruitment and differentiation of eosinophil progenitor cells in the bone marrow as well as the infiltration and survival of eosinophils at sites of inflammation are all regulated by IL-5 (47). Significant reduction in levels of IL-5 correlated with the reduced eosinophil peroxidase (EPO) activity in mice prophylactically treated with peptides P1, P2 and P3, and therapeutically with P1. The administration of Der p1-derived immunodominant peptide to the nasal mucosa of mice protected against airway inflammation with significant reductions in IL-5 levels and eosinophil infiltration into the airways following allergen challenge (32). However, a decrease in the levels of IL-5 cytokine did not result in protection against airway eosinophilia in P3 peptide-treated mice in prophylaxis, suggesting other factors contribute to the reduction in eosinophil numbers as reported earlier (48). It was interesting to note that in therapeutic regimen P2 peptide induced the decrease in IgE antibodies, showed higher ratios of IgG2a/IgG1 antibodies and IFN-γ/IL-4 cytokines than peptides P1 and P3. However, only P1 treated mice showed reduction in EPO activity, IL-5 and inflammatory cells infiltration in lung histological sections comparable to non-challenged control. Recently, it is becoming clear that immunopathogenesis of fungal infections cannot solely be explained in terms of TH1/TH2 balance, and the important role of TH17 pathway in fungal infections has been reported (49). Importantly, functional antagonism between TH17 and Treg has also been described (50). Thus, discrepancy observed between peptide-induced TH1/TH2 cytokine profile and resolution of lung inflammation in the present data suggest that other pathways (possibly TH17or Treg) could play significant role in Afu-induced allergies and need to be investigated.
It is important to mention that in previous studies peptide P1 induced in vitro histamine release from Afu-sensitized basophils of ABPA patients higher than peptides P2-P5, although significantly less than the 3wcf (19). Therefore, mechanism of protection and induction of TH1 cytokines by peptide P1 both prophylactically and therapeutically in in vivo conditions is difficult to explain and requires further studies. Akdis and colleagues have reported in their studies that histamine enhances secretion of IFN-γ by allergen-specific TH1 cells, whereas TH2 cytokines (IL-4, IL-13) were inhibited (51,52). Likewise, a recent study by Johansen et al demonstrated that antihistamine-treated CBA mice exhibit increased sensitization against bee-venom allergen extracts leading to increased levels of IgE antibodies while decreasing the secretion IgG2a antibodies (53). Further, redirection of the immune response towards TH1 has been demonstrated using whole-allergen (with histamine release potential) immunotherapy for several IgE-mediated allergies (54).
Since pulmonary eosinophilia was not inhibited by the peptides P2 and P3 in the therapeutic regimen and P3 in prophylactic regimen, it is likely that these peptides may not be effective for immunotherapy in the amounts administered and doses or routes of the peptide have to be optimized for the therapeutic or prophylactic potential.
Taken together, increased production of protective TH1 antibodies, elevated levels of TH1 cytokines and suppression of lung eosinophilia induced by Asp f1 peptides P1 and P2 prophylactically, and P1 therapeutically, establishes the usefulness of these peptides in the exploration of peptide-based protection against Afu-induced allergies. After establishing the protective efficacy of Asp f1 peptides in mice, we evaluated whether these results could be extrapolated to humans by predicting affinity of the peptides with various MHC class II molecules.
Peptides P1-P5 were predicted to be promiscuous in the present study as shown by their binding (medium binding affinity) to HLA-DR alleles such as HLA-DR2, HLA-DR4, HLA-DR5, HLA-DR7, and also with preponderant alleles such as HLADRB1*1501 (DR2) and HLADRB1*0401 (DR4) following SVM method. In Asian-Indian population HLADRB1*1501 while in Caucasian population both HLADRB1*1501 and HLADRB1*0401 cover ~60% of HLA-DRB1 allele frequency (55,56). Texier et al have identified T-cell epitopic regions from the major bee venom allergen, Phospholipase A-2, that efficiently bind to the predominant HLA-DRB1 molecules of Caucasian populations, suggesting as potential candidates for immunotherapy to bee-venom allergy (56). HLA association studies have revealed that the predisposition to develop ABPA is associated with HLA-DR2 and DR5, and possibly DR4 or DR7 (15). Therefore, predicted higher affinity of peptide epitope P1 than other peptides with HLA-DR5 suggests that this peptide may represent as a promising candidate for peptide-based intervention against allergic aspergillosis. However, experimental studies are required to confirm the predicted binding affinities to these HLA-alleles.
To conclude, our data suggests that P1, showing TH1 response in both prophylactic and therapeutic regimens in Balb/c mice challenged with Afu-allergens/antigens, is the most promising candidate and may be explored for the development of peptide-based immuno-therapeutics of allergic aspergillosis in humans.
Figures and Tables
Figure 1
Schematic representation of immunization/treatment schedule followed for prophylactic (A) and therapeutic (B) regimens with Asp f1 peptides P1-P3. i.n.: Intranasally, i.p.: Intraperitoneally, CFA: Complete Freund's adjuvant, IFA: Incomplete Freund's adjuvant, 3wcf: three-week culture filtrate.
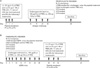
Figure 2
Afu-specific IgE and IgG antibody levels in sera after 10th day of prophylactic (A) and therapeutic (B) treatment with PBS or Asp f1 peptides P1-P3. Each regimen had 5 groups of mice: Non-challenged control, PBS-treated Afu-challenged (prophylactic) or ABPA (therapeutic), P1-treated, P2-treated and P3-treated mice. Data represents O.D. at 492 nm and error bars indicate the mean±SD of triplicate determinations with five mice per group, *p<0.05 versus PBS-treated ABPA/Afu challenged mice.

Figure 3
Afu-specific IgG2a and IgG1 antibody levels in sera of PBS-treated and peptide-treated mice groups (P1-P3) after 10th day of prophylactic (A) and therapeutic (B) regimen quantitated by indirect ELISA and their respective IgG2a/IgG1 ratios shown below the graph. Each regimen had 5 groups of mice: Non-challenged control, PBS-treated Afu-challenged (prophylactic) or ABPA (therapeutic), P1-treated, P2-treated and P3-treated mice. Data represents the mean±SD of triplicate determinations with five mice per group, *p<0.05 versus PBS-treated Afu challenged (prophylactic) or ABPA mice (therapeutic).

Figure 4
Levels of IFN-γ and IL-4 cytokines in splenic supernatants of PBS/peptide-treated mice groups (P1-P3) in prophylactic (A) and therapeutic regimen (B) quantitated by sandwich ELISA and their respective IFN-γ/IL-4 ratios shown below the graph. Each regimen had 5 groups of mice: Non-challenged control, PBS-treated Afu-challenged (prophylactic) or ABPA (therapeutic), P1-treated, P2-treated and P3-treated mice. Data represents the mean±SD of triplicate determinations with five mice per group, *p<0.05 versus PBS-treated Afu challenged (prophylactic) or ABPA mice (therapeutic).

Figure 5
Levels of IL-5 cytokine in mice splenic supernatants (A) and Lung EPO (Eosinophil Peroxidase) activity in lung homogenates (B) after prophylactic and therapeutic treatment with PBS or Asp f1 peptides P1-P3. Data represents the mean±SD of triplicate determinations with five mice per group, *p<0.05 versus PBS-treated Afu challenged mice, †p<0.05 versus PBS-treated ABPA mice.

Figure 6
Histopathological examination of the lung sections stained with hematoxylin and eosin (H&E) and observed at 20×, from the groups of PBS or Peptide-treated mice in prophylactic and therapeutic regimen. (A) PBS-treated Afu-challenged mice (B) PBS-treated ABPA mice (C) PBS-treated non-challenged control mice (D) PBS-treated non-challenged control mice (E) P1-treated naïve mice (F) P2-treated naïve mice (G) P3-treated naïve mice (H) P1-treated ABPA mice (I) P2-treated ABPA mice (J) P3-treated ABPA mice. Each micrograph represents the observations in triplicate (a minimum of 10 fields) made with four lung specimens of five mice per group.
ACKNOWLEDGEMENTS
We are grateful to, Institute of Genomics and Integrative Biology, a laboratory of Council of Scientific and Industrial Research, Govt. of India, for the facilities to carry out the present study. The financial support by the CSIR task forces COR0011 and SMM006 is gratefully acknowledged. We thank Ms Sneh Lata, research fellow at IMTECH, Chandigarh, for helping in SVM prediction of affinities of peptides. Ms Neelkamal Chaudhary is the recipient of a Senior Research Fellowship from the Council of Scientific and Industrial Research.
References
1. Tillie-Leblond I, Tonnel AB. Allergic bronchopulmonary aspergillosis. Allergy. 2005. 60:1004–1013.

2. Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, Denning DW, Crameri R, Brody AS, Light M, Skov M, Maish W, Mastella G. Participants in the Cystic Fibrosis Foundation Consensus Conference: Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003. 37:Suppl 3. S225–S264.
3. Zander DS. Allergic bronchopulmonary aspergillosis: an overview. Arch Pathol Lab Med. 2005. 129:924–928.

4. Eaton T, Garrett J, Milne D, Frankel A, Wells AU. Allergic bronchopulmonary aspergillosis in the asthma clinic. A prospective evaluation of CT in the diagnostic algorithm. Chest. 2000. 118:66–72.

5. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006. 6:761–771.

6. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007. 119:780–791.

7. Ajduk J, Marinic I, Aberle N, Rabatic S, Gagro A. Effect of house dust mite immunotherapy on transforming growth factor beta1-producing T cells in asthmatic children. Ann Allergy Asthma Immunol. 2008. 100:314–323.
8. Pfaar O, Klimek L. Anaphylaxis--life-threatening allergic reactions. MMW Fortschr Med. 2008. 150:35–39.
9. Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2008. 100:256–263.

10. Senti G, Johansen P, Martinez Gomez J, Prinz Varicka BM, Kündig TM. Efficacy and safety of allergen-specific immunotherapy in rhinitis, rhinoconjunctivitis, and bee/wasp venom allergies. Int Rev Immunol. 2005. 24:519–531.

11. Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009. 123:735–746.

12. Lizaso MT, Tabar AI, García BE, Gómez B, Algorta J, Asturias JA, Martinez A. Double-blind, placebo-controlled Alternaria alternata immunotherapy: in vivo and in vitro parameters. Pediatr Allergy Immunol. 2008. 19:76–81.
13. Malling HJ, Djurup R. Diagnosis and immunotherapy of mould allergy. VII. IgG subclass response and relation to the clinical efficacy of immunotherapy with Cladosporium. Allergy. 1988. 43:60–70.
14. Tabar AI, Lizaso MT, García BE, Gómez B, Echechipía S, Aldunate MT, Madariaga B, Martínez A. Double-blind, placebo-controlled study of Alternaria alternata immunotherapy: clinical efficacy and safety. Pediatr Allergy Immunol. 2008. 19:67–75.

15. Chauhan B, Knutsen A, Hutcheson PS, Slavin RG, Bellone CJ. T cell subsets, epitope mapping, and HLA-restriction in patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 1996. 97:2324–2331.

16. Kurup VP, Banerjee B, Murali PS, Greenberger PA, Krishnan M, Hari V, Fink JN. Immunodominant peptide epitopes of allergen, Asp f1 from the fungus Aspergillus fumigatus. Peptides. 1998. 19:1469–1477.

17. Madan T, Arora N, Sarma PU. Ribonuclease activity dependent cytotoxicity of Asp fl, a major allergen of A. fumigatus. Mol Cell Biochem. 1997. 175:21–27.
18. Madan T, Arora N, Sarma PU. Identification and evaluation of a major cytotoxin of A. fumigatus. Mol Cell Biochem. 1997. 167:89–97.
19. Madan T, Priyadarsiny P, Vaid M, Kamal N, Shah A, Haq W, Katti SB, Sarma PU. Use of a synthetic peptide epitope of Asp f1, a major allergen or antigen of Aspergillus fumigatus, for improved immunodiagnosis of allergic bronchopulmonary aspergillosis. Clin Diagn Lab Immunol. 2004. 11:552–558.

20. Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of cytotoxic T cell responses directed to human leucocyte antigen Class I restricted epitopes from the Aspergillus f16 allergen. Clin Exp Immunol. 2005. 140:81–91.

21. Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of Th1 T cell responses directed to a HLA Class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol. 2005. 139:257–267.

22. Svirshchevskaya EV, Alekseeva LG, Titov VM, Frolova EG, Sapozhnikov AM. Determination of T and B Cell Epitopes of Aspergillus fumigatus Ribotoxin and Heat Shock Protein. Russ J Immunol. 1998. 3:61–68.
23. Arruda LK, Mann BJ, Chapman MD. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992. 149:3354–3359.
24. Moser M, Crameri R, Menz G, Schneider T, Dudler T, Virchow C, Gmachl M, Blaser K, Suter M. Cloning and expression of recombinant Aspergillus fumigatus allergen I/a (rAsp f I/a) with IgE binding and type I skin test activity. J Immunol. 1992. 149:454–460.
25. Kamal N, Chowdhury S, Madan T, Sharma D, Attreyi M, Haq W, Katti SB, Kumar A, Sarma PU. Tryptophan residue is essential for immunoreactivity of a diagnostically relevant peptide epitope of A. fumigatus. Mol Cell Biochem. 2005. 275:223–231.

26. Svirshchevskaya E, Frolova E, Alekseeva L, Kotzareva O, Kurup VP. Intravenous injection of major and cryptic peptide epitopes of ribotoxin, Asp f1 inhibits T cell response induced by crude Aspergillus fumigatus antigens in mice. Peptides. 2000. 21:1–8.

27. Bhasin M, Raghava GP. SVM based method for predicting HLA-DRB1*0401 binding peptides in an antigen sequence. Bioinformatics. 2004. 20:421–423.

28. Zhang GL, Srinivasan KN, Veeramani A, August JT, Brusic V. PREDBALB/c: a system for the prediction of peptide binding to H2d molecules, a haplotype of the BALB/c mouse. Nucleic Acids Res. 2005. 33:W180–W183.

29. Bhasin M, Raghava GP. Prediction of CTL epitopes using QM, SVM and ANN techniques. Vaccine. 2004. 22:3195–3204.

30. Madan T, Kishore U, Singh M, Strong P, Clark H, Hussain EM, Reid KB, Sarma PU. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001. 107:467–475.

31. Astori M, von Garnier C, Kettner A, Dufour N, Corradin G, Spertini F. Inducing tolerance by intranasal administration of long peptides in naive and primed CBA/J mice. J Immunol. 2000. 165:3497–3505.

32. Hall G, Houghton CG, Rahbek JU, Lamb JR, Jarman ER. Suppression of allergen reactive Th2 mediated responses and pulmonary eosinophilia by intranasal administration of an immunodominant peptide is linked to IL-10 production. Vaccine. 2003. 21:549–561.

33. Nigam S, Ghosh PC, Sarma PU. Altered immune response to liposomal allergens of Aspergillus fumigatus in mice. Int J Pharm. 2002. 236:97–109.

34. Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968. 97:77–89.
35. Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005. 35:52–58.

36. Hoyne GF, O'Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993. 178:1783–1788.

37. Kay AB, Larché M. Allergen immunotherapy with cat allergen peptides. Springer Semin Immunopathol. 2004. 25:391–399.

38. Yoshitomi T, Nakagami Y, Hirahara K, Taniguchi Y, Sakaguchi M, Yamashita M. Intraoral administration of a T-cell epitope peptide induces immunological tolerance in Cry j 2-sensitized mice. J Pept Sci. 2007. 13:499–503.

39. Helbling A, Reimers A. Immunotherapy in fungal allergy. Curr Allergy Asthma Rep. 2003. 3:447–453.

40. Crameri R, Weichel M, Flückiger S, Glaser AG, Rhyner C. Fungal allergies: a yet unsolved problem. Chem Immunol Allergy. 2006. 91:121–133.

41. Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008. 145:58–86.

42. Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988. 140:1022–1027.
43. Kozutsumi D, Tsunematsu M, Yamaji T, Murakami R, Yokoyama M, Kino K. Cry-consensus peptide, a novel peptide for immunotherapy of Japanese cedar pollinosis, induces Th1-predominant response in Cry j 1-sensitized B10.S mice. Biol Pharm Bull. 2006. 29:2506–2509.

44. von Garnier C, Astori M, Kettner A, Dufour N, Heusser C, Corradin G, Spertini F. Allergen-derived long peptide immunotherapy down-regulates specific IgE response and protects from anaphylaxis. Eur J Immunol. 2000. 30:1638–1645.

45. Furin MJ, Norman PS, Creticos PS, Proud D, Kagey-Sobotka A, Lichtenstein LM, Naclerio RM. Immunotherapy decreases antigen-induced eosinophil cell migration into the nasal cavity. J Allergy Clin Immunol. 1991. 88:27–32.

46. Rak S, Löwhagen O, Venge P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen-allergic patients. J Allergy Clin Immunol. 1988. 82:470–480.

47. Hamelmann E, Wahn U, Gelfand EW. Role of the Th2 cytokines in the development of allergen-induced airway inflammation and hyperresponsiveness. Int Arch Allergy Immunol. 1999. 118:90–94.

48. Kurup VP, Murali PS, Guo J, Choi H, Banerjee B, Fink JN, Coffman RL. Anti-interleukin (IL)-4 and -IL-5 antibodies downregulate IgE and eosinophilia in mice exposed to Aspergillus antigens. Allergy. 1997. 52:1215–1221.

50. Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005. 201:169–171.
51. Jutel M, Klunker S, Akdis M, Malolepszy J, Thomet OA, Zak-Nejmark T, Blaser K, Akdis CA. Histamine upregulates Th1 and downregulates Th2 responses due to different patterns of surface histamine 1 and 2 receptor expression. Int Arch Allergy Immunol. 2001. 124:190–192.

52. Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001. 413:420–425.

53. Johansen P, Senti G, Maria Martínez Gómez J, Kündig TM. Medication with antihistamines impairs allergen-specific immunotherapy in mice. Clin Exp Allergy. 2008. 38:512–519.

54. Jutel M, Blaser K, Akdis CA. Histamine receptors in immune regulation and allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2006. 26:245–259.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download