Abstract
DNA immunization induces B and T cell responses to various pathogens and tumors. However, these responses are known to be relatively weak and often transient. Thus, novel strategies are necessary for enhancing immune responses induced by DNA immunization. Here, we demonstrated that co-immunization of influenza virus nucleoprotein (NP) gene significantly enhances humoral and cell-mediated responses to codelivered antigens in mice. We also found that NP DNA coimmunization augments in vivo proliferation of adoptively transferred antigen-specific CD4 and CD8 T cells, which enhanced protective immunity against tumor challenge. Our results suggest that NP DNA can serve as a novel genetic adjuvant in cocktail DNA vaccination.
DNA vaccines provide several advantages over live attenuated or vectored vaccines; relatively safe, easy to manufacture, easy formulation of multivalent vaccines, and the induction of type 1 CD4+ T cell-mediated immune responses (1-3). However, it has been shown that DNA vaccine alone is unable to confer complete protection against some infectious pathogens, due to its relatively weak immunogenicity, particularly in large animal model (1,4,5). To overcome the poor immunogenicity, codelivery of plasmid DNA encoding various adjuvant molecules has been used to augment the immune responses elicited by DNA immunization. For example, cytokines such as IL-2 (6), IL-12 (7,8), and GM-CSF (9,10) have been shown to efficiently enhance antigen-specific immune responses induced by DNA vaccine. Co-stimulatory molecules, such as B7.1, B7.2 and CD40L, have been also found to increase antigen-specific T-cell responses by enhancing antigen-presenting cells' (APC) functions (11-13). However, the use of cytokine/co-stimulatory molecules as genetic adjuvants could raise safety concern, since relatively little information is available on their long-term safety in humans (14). Also, DNA vaccines formulated in either a solution containing a nonionic blocked copolymer adjuvant (CRL 1005) or in monophosphoryl lipid A adsorbed onto aluminium phosphate (MPL/alum) appeared to enhance vaccine-elicited cellular immune response against co-delivered antigens like SIV Gag (15). DNA itself could be used to enhance immunogenicity if it contains cytidine-phosphate-guanosine (CpG) motif, which is known to stimulate innate immune system (16,17). Indeed, HIV-1 specific cytotoxic T-lymphocyte (CTL) response and humoral response are enhanced by the coimmunization of DNA containing the CpG motif (18,19).
One of the most beneficial things about DNA vaccination is the fact that several plasmid vectors can be easily formulated into a single inoculum to induce multivalent immune responses to various antigens simultaneously. It has been well demonstrated that immunization of a mixture of plasmids, called cocktail DNA vaccination, induces broad immune responses to multiple-antigens in DNA vaccine models (20). However, it remains to be elucidated whether the administration of a cocktail DNA vaccine results in immune interference or enhancement to each other. For example, Kjerrstrom et al. reported that immunization with a mixture of plasmids encoding the HIV regulatory genes tat, rev, and nef resulted in reduced T-cell responses to Rev and Nef, compared to those observed in single gene immunization (21). These results suggest that specific immune response to an antigen may inhibit other responses to codelivered antigens in a multigene vaccine. In contrast, another study by Grifantini et al. showed that poor immunogenicity of plasmid encoding major merozoite surface protein (MSP)-1 were enhanced in cocktail DNA vaccination compared with those in a single plasmid DNA immunization (22).
In this study, we found that coimmunization of the NP gene significantly enhances specific humoral and cellular immune responses to various DNA-encoded antigens. In addition, NP DNA coimmunization elicited faster and vigorous in vivo proliferation of adoptively transferred CD8 T cells and CD4 T cells specific for codelivered ovalbumin (OVA) antigen, and more efficient protection against modified tumor challenge than single OVA DNA immunization. Our findings suggest that the NP gene could function as a genetic adjuvant.
MDCK cells were infected with influenza A/PR/8/34 virus and influenza A/Jap/57 viruses, respectively, and total RNA was isolated. The NP gene of influenza A/PR/8/34 and the hemagglutinin (HA) gene of Influenza A/Jap/57 were then obtained by conducting RT-PCR with the isolated RNA. These NP and HA genes, cleaved by XhoI/XbaI and KpnI/Xho I, were inserted into pTV2 vector (9) to prepare pTV-NP and pTV-HA, respectively. pTX-GE (6), cleaved by MluI and HpaI, was inserted into pTV2 vector to prepare pTV-GE. pTV-gDsE2t expressing truncated HCV E2 protein is described elsewhere (9). Chicken OVA cDNA from Tc-OVA vector was amplified by PCR and inserted into pTV2 vector to prepare pTV-OVA. Each plasmid DNA was grown in E. coli and then purified using endotoxin-free kits (QIAGEN, Valencia, CA). Endotoxin levels were measured using Limulus Amebocyte Lysate assay (Sigma, St. Louis, MO) and were typically negligible in all DNA preparations. The expression of NP was identified using anti-Flu (A/PR/8/34) mouse serum by the radioimmunoprecipitation method in transient transfection assay. The expressions of pTV-OVA and pTV-GE were confirmed by Western blot analysis as described previously (6).
Six to seven week-old female mice (BALB/c or C57BL/6), purchased from crSLC, Japan, were used for the DNA immunization experiments. Plasmids were dissolved in PBS at a concentration of 1 mg/ml, and a total of 100 µg DNA was intramuscularly injected into the tibials muscle in both hind legs of BALB/c mice (50 µl in each leg). Four weeks after the first immunization, a booster immunization was performed at the same region using the same DNA vaccines. For coimmunization with OVA DNA and NP DNA, C57BL/6 mice were used in intramuscular immunization. In cases of single DNA immunization, 50 µg of pTV empty vector were used to adjust the total amount of injected DNA to 100 µg.
HA and NP protein were partially purified from an Influenza bulk vaccine (LG Chemical Co. Ltd., Korea) using a Con-A Cephalos column (Amersham Biosciences, Piscataway, NJ), according to the manufacturer's instruction. Each protein solution was separated by SDS-PAGE. Gels corresponding to NP and HA protein bands were cut out and electroeluted for use as NP and HA antigens in ELISA. Antibody responses to HIV-1 structural protein and HCV E2 protein were measured by ELISA using HIV-1 viral lysate (6) and hghE2t protein (9). Mice sera were collected 4 weeks after final immunization to determine the anti-HA, anti-Env or anti-E2 response. ELISA was used to detect antibody response, as described previously (23).
CTL activity was measured by using a conventional 51Cr release assay. Four weeks after the booster immunization, splenocytes of mice in each group were prepared and maintained in a CTL analysis culture medium (RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 50 µM β-mercaptoethanol, and 10 U/ml recombinant murine IL-2). To stimulate NP-specific and HA-specific lymphocytes, cells were incubated with 7 µM of each peptide at 37℃ in 5% CO2 for 6 days. P815 (H-2d) target cells were pulsed with 5µM of NP (TYQRTRALV) or HA peptide (LYQNVGTYV), labeled with 51Cr, and reacted with the stimulated effector cells to measure cytotoxicity. To stimulate the HIV env-specific CTLs, the irradiated splenocytes of non-immunized BALB/c mice infected with a recombinant vaccinia virus expressing HIV-1 (IIIB) env (National Institutes of Health AIDS Research and Reference Reagent Program) were used as a stimulator. The splenocytes of the immunized mice were cultivated for 6 days together with the stimulator cells, and then reacted with V3 peptide (RIQRGPGRAFVTIGK) pulsed-P815 target cells. HCV E2-specific CTL response was measured as described previously (24).
IFN-γ ELISPOT assay was performed as described previously (25). Briefly, splenocytes were serially two-fold diluted starting with 4×105 cells per well on IFN-γ capture mAb (5 µg/ml, BD Pharmingen, San Diego, CA)-coated nitrocellulose 96-well plates (Millipore, Bedford, MA), in triplicate, and 10 µM each of OVA 257-264 (SIINFEKL, H-2Kb-restricted), OVA 323-339 peptide (ISQAVHAAHAEINEAGR, I-Ab restricted), 5 µM of NP peptide (ASNENMETM, H-2Db-restricted), or HIV-1 V3 peptide (RIQRGPGRAFVTIGK, Dd-restricted) was then added. After incubation at 37℃ in 5% CO2 for 24 h, the plates were washed 5 times with PBS containing 0.05% Tween 20 (PBST) and treated with 2.5 µg/ml of biotin-conjugated anti-IFN-γ detection mAb (BD Pharmingen) followed by streptavidin-conjugated alkaline phosphatase. The BCIP/ NBT substrates were then added to the plates, and the reaction was terminated with excess water when blue spots were observed after several minutes. The number of spots was counted using an ELISPOT reader (AID).
TCR-transgenic T cells from OT-I or OT-II mice (26), which are specific to the OVA 257-264 or the OVA 323-339, respectively, were used as donor cells in adoptive transfer experiments. A single cell suspension was obtained from the lymph nodes of 6-week-old OT-I or OT-II mice, and treated with anti-HSA (J11d), anti-B220, anti-MHC class II, and anti-CD4 microbead antibodies for CD8 T cells (or anti-CD8 for CD4 T cells) at 4℃ for 30 minutes, and then treated with a rabbit complement at 37℃ for 45 minutes to obtain OT-I (or OT-II) cells at a purity of more than 95%. Microbeads were obtained from Miltenyi Biotec (Auburn, CA). The purified cells were resuspended in PBS at a concentration of 2×107 cells/ml, and labeled with 5 µM of CFSE (Molecular Probes, Eugene, OR) to trace cell division in vivo. The labeled cells were then transferred into 6 to 7-week-old female C57BL/6 mice. After 24h, the recipient mice were intramuscularly injected with plasmid DNA, and cells from the draining lymph nodes of each mouse were prepared 9 days after immunization. Cells were stained with PerCP-conjugated anti-CD4 or CD8 mAb (BD Pharmingen, SanDiego, CA) and PE-conjugated Vα2 mAb (BD Pharmingen) at 4℃ for 15 minutes. Finally, 50,000~100,000 cells were collected using FACSCalibur (BD Biosciences, San Jose, CA) and analyzed with CellQuest software (BD Biosciences).
OVA-specific CD8 T cells from the lymph nodes of six-week-old OT-I mice were purified by negative selection using MACS, as described above, and adoptively transferred into normal mice. At day 1, each plasmid DNA was intramuscularly injected into the mice. The DNA-immunized mice were subcutaneously injected with EG-7 tumor cells (5×105 cells/mouse in 100 µl of PBS) expressing OVA after 28 days. The tumor size was regularly checked using microcaliper at the indicated time points. Percentage of tumor incidence was represented as the population of mice bearing palpable tumors (tumor volume>40 mm3).
DNA immunization with a mixture of plasmids encoding influenza HA and NP has been known to induce more effective protection against influenza virus infection than that with a single plasmid (27). However, it remains unknown whether the enhanced protection is associated with varied breadth and strength of immune responses. In this study, we evaluated the possibility that immune response to specific antigen elicited by DNA immunization could be modulated by coimmunized DNA in cocktail DNA vaccine model. To this end, pTV-NP, pTV-HA, or a mixture of pTV-HA and pTV-NP were injected into female BALB/c mice twice at four-week interval. Interestingly, anti-HA antibodies in mice immunized with pTV-HA DNA were nearly detectable, but were significantly enhanced by coinjection with pTV-NP DNA (p<0.005, Fig. 1A). Similarly, CTL response to HA antigen, but not to NP antigen, were enhanced by coinjection of pTV-HA and pTV-NP compared with by single DNA immunization (Fig. 1B). As a control, mice immunized with pTV-NP DNA alone did not induce HA-specific antibody and CTL immune responses, and vice versa (Fig. 1A, and data not shown), indicating the absence of cross-reactivity between these two antigens.
To investigate whether this adjuvanticity of influenza NP DNA is broadly applicable to other antigen in DNA vaccine model, a plasmid DNA encoding HIV gag-env (pTV-GE) or HCV E2 (pTV-gDsE2t) was injected with or without pTV-NP twice into female BALB/c mice at four-week interval. Antibody and CTL responses to HIV Env and HCV E2 were determined at 4 weeks after booster immunization using serum and splenocytes from the immunized mice, respectively. As expected, anti-Env and anti-E2 antibody responses were significantly enhanced by coinjection with pTV-NP DNA (p<0.005 and p<0.05, respectively, Fig. 1A). Similarly, Env- and E2-specific CTL responses determined by using a conventional 51Cr release assay were enhanced by codelivery of pTV-NP DNA (Fig. 1B). Immunization of pTV-NP DNA alone did not induce Env- or E2-specific immune response, and vice versa. Furthermore, coimmunization of pTV-NP-R which has NP gene in a reverse orientation failed to increase immune response to codelivered antigen (data not shown), suggesting that the enhancement is not presumably due to potent stimulating CpG motifs within NP gene. On the other hand, NP-specific antibody and CTL responses were not affected by coinjection with pTV-GE DNA or pTV-gDsE2t DNA (data not shown). Since endotoxin is able to enhance DNA-encoded antigen-specific immune responses, the endotoxin level in NP DNA purified by ultrapure endotoxin-free DNA purification kit (QIAGEN) was measured and shown to be less than 3 endotoxin units (EU)/µg DNA for in vivo use. In addition, there was no significant difference in the endotoxin level in each plasmid DNA. Thus, it is unlikely that endotoxin contamination contributes to the effect of NP DNA on immune enhancement. These results clearly indicate that NP gene specifically enhances antibody and CTL responses to a broad range of codelivered antigens in DNA vaccination model. It is worth noting that the individual difference of CTL response appeared to be less than of antibody response because splenocytes were pooled for CTL assay.
The longevity of immune responses induced by vaccination is one of the most critical parameters in determining the efficacy of vaccines. To determine whether the coadministration of NP DNA elicits sustained immune responses specific to codelivered antigen, two groups of mice were injected twice at 0 and 6 weeks with pTV-GE with or without pTV-NP. As shown in Fig. 1C, the frequency of HIV Env-specific IFN-γ-producing cells determined by ELISPOT assay was significantly increased by codelivery of NP DNA at 5 weeks after the first immunization and 4 and 8 weeks after the booster immunization (10 and 14 weeks, p<0.005 and p<0.001, respectively). These results suggest that NP DNA coimmunization not only enhances antibody and CTL responses but also prolongs memory T-cell immunity to specific antigen induced by DNA immunization.
To define dose-dependence in immune enhancement by NP DNA, female C57BL/6 mice were immunized with 50 µg pTV-OVA plus various concentration of NP DNA (from 0 to 100 µg) and then the induction of OVA-specific CD4 and CD8 T cells was quantitatively determined by ELISPOT assay using OVA (323-339: I-Ab-restricted) and OVA (257-264: H-2Kb-restricted) peptide, respectively, at 4 weeks after booster immunization. As shown in Fig. 2A, coinjection of pTV-OVA even with 5 µg of pTV-NP induced higher numbers of OVA-specific IFN-γ-producing CD4 and CD8 T cells than that by pTV-OVA alone and peak responses were observed with 50 µg of NP DNA (Fig. 2A). It was previously reported that the frequency of memory T cells is dependent on the initial burst size of the primary effector cells (28). Thus, it is likely that enhancement of memory T cell immunity by NP DNA coinjection is due to increase of the primary expansion of codelivered antigen-specific T cells.
To investigate the adjuvant effect of NP DNA on proliferation of antigen-specific T cells, purified naive OVA-specific OT-I and OT-II cells were labeled with the fluorescent dye, CFSE, and then adoptively transferred into female C57BL/6 mice. One day later, mice were immunized with pTV-NP, or pTV-OVA, or a mixture of pTV-NP and pTV-OVA. At 9 days post-immunization, draining lymph node cells from the immunized mice were analyzed for in vivo proliferation of donor OT-I and OT-II cells by flow cytometry. OT-I cells from mice coimmunized with pTV-NP and pTV-OVA appeared to proliferate more extensively than those with pTV-OVA alone, although both of them displayed the same number of division cycles (i.e., more than five times) (Fig. 2B). In particular, proliferation of transferred OT-II cells was notably enhanced in the presence of pTV-NP (Fig. 2C). It is worth noting that OVA DNA immunization alone is able to induce weak but significant proliferation of adoptively transferred OT-I cells, but not OT-II cells. These results are consistent with reported that the proliferation of naive CD4 T cells requires 100-fold higher levels of antigenic stimulation than that required for naïve CD8 T cell proliferation in vitro and in vivo (29,30). As a negative control, transferred OT-I and OT-II cells did not proliferate when mice were immunized with pTV-NP alone (Fig. 2B).
It has been known that not only HA-specific antibody and CTL responses but also NP-specific CTL response are important for protection against influenza virus infection (31,32). Thus, if the protective immunity induced by NP+HA codelivery is stronger than that by NP or HA single immunization, it is not clear whether the protection is due to either the broad immunity to influenza NP and HA or the enhancement of HA-specific immune response by codelivery of NP DNA as adjuvant. Accordingly, it is likely that the use of tumor challenge model instead of influenza challenge is more appropriate to show the adjuvant effect of NP DNA in DNA immunization model. Therefore, to determine whether the increased frequency of antigen-specific T cells by codelivery of NP DNA correlates with in vivo protection against tumor challenge, C57BL/6 mice were adoptively transferred with naive OT-I cells and then intramuscularly injected with pTV-OVA DNA in the presence or absence of NP DNA one day after adoptive transfer. At 28 days post-immunization, the immunized mice were challenged with EG-7 tumor cells expressing OVA and tumor growth was examined. As expected, tumor growth was significantly delayed and tumor incidence was only 17% of mice coimmunized with pTV-NP and pTV-OVA (p<0.01) (Fig. 3). Interestingly, pTV-OVA-immunized mice also showed delayed tumor growth, compared with the pTV-NP-immunized mice as a control. However, pTV-OVA-immunized group eventually exhibited the same tumor incidence as control mice (50%) at day 30 after tumor challenge. Taken together, these results suggest that the enhanced proliferation and frequency of antigen-specific T cells induced by the codelivery of NP DNA increases protection against tumor challenge.
It has been shown that influenza NP DNA immunization induces relatively potent humoral and cellular immune responses to NP protein, suggesting that NP has strong immunogenicity (33,34). In general, it is believed that immune response is decreased by antigen competition when highly immunogenic antigens are coadministered. Upon virus infection, immune responses to dominant epitopes of the viral antigen are known to interfere with the induction of subdominant and/or cryptic epitope-specific responses (35-37). Moreover, it has been suggested that coinfection with highly immunogenic viruses causes immune interference rather than immune enhancement (35). However, in the present study, we showed that NP gene can serve as a genetic adjuvant in multigene DNA immunization models, augmenting codelivered antigen-specific antibody and T cell responses. Since our results somehow contrast with the previous observations in a virus infection model, it is likely that there are marked differences in the outcomes of antigen-specific immune competition between virus infection and DNA vaccine model, which would be derived from the different environments of both systems, such as location and expression level of antigen, the presence of CpG motif (18,38), and the replication ability of the vector. As agreed with our results, when the MN V3 region of HIV-1 was fused with HBV surface antigen (HBsAg), the V3-specific antibody and CTL responses were enhanced, as compared with the results of immunization with MN gp160 DNA (39). This study suggests that highly immunogenic antigen like HBsAg acts as "a fused adjuvant", which augments and accelerates cellular and humoral immune response against other antigens in DNA vaccine model.
Like genetic modulators such as cytokines and costimulatory molecules, it is possible that the adjuvant effect of NP gene is due to the induction of a beneficial microenvironment for the optimal activation of codelivered antigen-specific naive T cells by potent NP-specific immunity. NP-specific T cells induced by NP DNA coinjection cause the activation and maturation of cognate or noncognate dendritic cells either by providing CD40L signaling or by secreting immunostimulatory soluble factors, as described in previous reports (40). The licensed dendritic cells in the microenvironment may in turn enhance the magnitude of T-cell responses specific for codelivered target antigens. In addition, given that the induction of CTL responses depends on cognate CD4 T cell help in a DNA vaccine model, the CD4 T cell responses which were significantly enhanced by codelivery of pTV-NP might be involved in the effect of NP gene product. Thus, it is likely that potent NP-specific T-cell immunity decreases the threshold levels for activation and proliferation of naive CD4 T cells, which is known to be more difficult than those of naive CD8 T cells (30). CD8+ CTLs are known to be capable of potent anti-tumor immune response (41). Our results show that enhancement of OVA-specific CD8 T cell frequency and proliferation induced by NP DNA codelivery promotes protective immunity against tumor challenge. The data suggested that the ability of NP DNA to enhance broad immune responses make it a potent adjuvant for enhancing protective efficacy requiring humoral or cellular immunity.
In summary, we show that the NP gene has a potent genetic adjuvant effect on a broad range of codelivered antigens. This information will be of help on the design of formulations in the effective cocktail DNA vaccines, considering the immunogenic potential of each codelivered plasmid-encoded antigen.
Figures and Tables
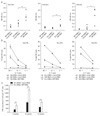 | Figure 1Increased antigen-specific antibody and CTL responses by NP DNA coimmunization. The plasmid vectors encoding the influenza viruses HA (A/Jap/57), HIV Env (HXBc2) and HCV E2 (genotype 1b) were mixed with NP (A/PR/8/34) or mock DNA, and then intramuscularly injected twice, at four-week interval, into female BALB/c mice. Antibody (A) and CTL responses (B) to HA, HIV Env, and HCV E2 were analyzed 4 weeks after the boost immunization. (A) Sera from the immunized mice were diluted to 1/100 and antibody responses were determined by ELISA assay. Circles represent O.D. at 405 nm and the bars are the means of four mice per group. Data are representative of two independent experiments. *p<0.05, **p<0.005 (B) Splenocytes from two mice per group were pooled and the specific killing of HA peptide-loaded P815 target cells was determined by standard 51Cr release assay. Killing of P815 target cells without HA peptide was <2%. rVV Env-infected naive syngeneic splenocytes and P815 cells were used as stimulator and target cells, respectively, for determining HIV Env-specific CTL response. An E2-expressing CT26 cell line was used as stimulator and target cells for measuring HCV E2-specific CTL activity. Non-specific killing of target cells without Env or E2 was <5%. Similar data were obtained in three separate experiments. (C) HIV Env-specific T-cell responses were measured by ELISPOT assay. Each group of mice were immunized twice with pTV-GE in the absence or presence of pTV-NP at 0 and 6 weeks. At the indicated time points, pooled splenocytes were prepared and Env-specific IFN-γ responses to V3 peptide were analyzed. Values represent the means±s.d. of triplicate cultures in one experiment. Similar data were obtained in three separate experiments. **p<0.005, ***P<0.001, Statistical significance was determined using the Student t test. |
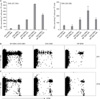 | Figure 2Increased frequency and proliferation of OVA-specific CD4 and CD8 T cells by coimmunization of NP DNA. (A) Plasmid DNA encoding OVA was mixed with different doses of NP DNA(5, 20, 50, and 100 µg) or mock DNA, and then intramuscularly injected into female C57BL/6 mice. At 4 weeks after booster DNA immunization, pooled splenocytes were prepared from two mice per group. Cells were stimulated with OVA (257-264: H-2Kb-restricted epitope) or OVA (323-339: class II-restricted epitope) peptides and analyzed for IFN-γ-producing T cells by ELISPOT assay. Values represent the means ± s.d. of triplicate cultures in one experiment. The experiment was repeated three times and produced similar results. (B, C) CFSE-labeled naive OT-I and OT-II cells (2×106) were adoptively transferred into C57BL/6 mice and then one day later OVA DNA was intramuscularly injected with or without NP DNA. At day 9 post-immunization, pooled draining inguinal lymph nodes from two mice per group were analyzed by measuring the in vivo proliferation of (B) OT-I and (C) OT-II cells by flow cytometry. Live lymphocytes were gated according to forward and side light scatter profiles. Data are representative of three independent experiments that produced similar results. |
 | Figure 3NP DNA coinjection enhances protection against tumor challenge. Naive. OT-I cells (2×106) were adoptively transferred into C57BL/6 mice and the DNA encoding OVA was intramuscularly injected with or without NP DNA one day later (n=12). At 28 days post-immunization, mice were s.c. inoculated with 5×105 EG-7 tumor cells. Tumor sizes were measured using microcaliper in two dimensions. The populations of mice bearing palpable tumors (tumor volume>40 mm3) are presented as the percentage of tumor incidence. Coimmunization of OVA DNA and NP DNA showed the reduced level of tumor incidence (p<0.01, Student t test). |
ACKNOWLEDGEMENTS
We express our thanks to Sang Chun Lee for animal care, and are indebted to Dr. W. R. Heath (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for providing OT-I and OT-II TCR-transgenic mice.
This work was supported by the KOSEF grant funded by the Korea government (MOST) (NO. M1053405001-06N3405-00110), and by grant from LG Life Sciences LTD.
References
3. Beláková J, Horynová M, Krupka M, Weigl E, Raska M. DNA vaccines: are they still just a powerful tool for the future? Arch Immunol Ther Exp (Warsz). 2007. 55:387–398.
4. Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol Res. 2008. 42:219–232.

5. Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther. 2006. 17:1051–1061.

6. Lee AH, Suh YS, Sung YC. DNA inoculations with HIV-1 recombinant genomes that express cytokine genes enhance HIV-1 specific immune responses. Vaccine. 1999. 17:473–479.

7. Suh YS, Ha SJ, Lee CH, Sin JI, Sung YC. Enhancement of VP1-specific immune responses and protection against EMCV-K challenge by co-delivery of IL-12 DNA with VP1 DNA vaccine. Vaccine. 2001. 19:1891–1898.

8. Yu DH, Li M, Hu XD, Cai H. A combined DNA vaccine enhances protective immunity against Mycobacterium tuberculosis and Brucella abortus in the presence of an IL-12 expression vector. Vaccine. 2007. 25:6744–6754.

9. Lee SW, Cho JH, Sung YC. Optimal induction of hepatitis C virus envelope-specific immunity by bicistronic plasmid DNA inoculation with the granulocyte-macrophage colony-stimulating factor gene. J Virol. 1998. 72:8430–8436.

10. Maksaereekul S, Dubie RA, Shen X, Kieu H, Dean GA, Sparger EE. Vaccination with vif-deleted feline immunodeficiency virus provirus, GM-CSF, and TNF-alpha plasmids preserves global CD4 T lymphocyte function after challenge with FIV. Vaccine. 2009. 27:3754–3765.

11. Gomez CE, Najera JL, Sanchez R, Jimenez V, Esteban M. Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus vectors MVA and NYVAC expressing HIV antigens. Vaccine. 2009. 27:3165–3174.

12. Maue AC, Waters WR, Palmer MV, Whipple DL, Minion FC, Brown WC, Estes DM. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine. 2004. 23:769–779.

13. Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, Kornbluth RS. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006. 80:1762–1772.

14. Salgaller ML, Lodge PA. Use of cellular and cytokine adjuvants in the immunotherapy of cancer. J Surg Oncol. 1998. 68:122–138.

15. Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002. 415:331–335.

16. Chaudhry UI, Kingham TP, Plitas G, Katz SC, Raab JR, DeMatteo RP. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 2006. 66:10497–10504.

17. Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009. 182:1846–1853.

18. Kojima Y, Xin KQ, Ooki T, Hamajima K, Oikawa T, Shinoda K, Ozaki T, Hoshino Y, Jounai N, Nakazawa M, Klinman D, Okuda K. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine. 2002. 20:2857–2865.

19. Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996. 273:352–354.

20. Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, Sambor A, Chakrabarti BK, Huang Y, Bailer R, Koup RA, Mascola JR, Nabel GJ, Letvin NL. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005. 79:2956–2963.

21. Kjerrstrom A, Hinkula J, Engström G, Ovod V, Krohn K, Benthin R, Wahren B. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology. 2001. 284:46–61.

22. Grifantini R, Finco O, Bartolini E, Draghi M, Del Giudice G, Kocken C, Thomas A, Abrignani S, Grandi G. Multi-plasmid DNA vaccination avoids antigenic competition and enhances immunogenicity of a poorly immunogenic plasmid. Eur J Immunol. 1998. 28:1225–1232.

23. Sin JI, Sung JH, Suh YS, Lee AH, Chung JH, Sung YC. Protective immunity against heterologous challenge with encephalomyocarditis virus by VP1 DNA vaccination: effect of coinjection with a granulocyte-macrophage colony stimulating factor gene. Vaccine. 1997. 15:1827–1833.

24. Song MK, Lee SW, Suh YS, Lee KJ, Sung YC. Enhancement of immunoglobulin G2a and cytotoxic T-lymphocyte responses by a booster immunization with recombinant hepatitis C virus E2 protein in E2 DNA-primed mice. J Virol. 2000. 74:2920–2925.

25. Power CA, Grand CL, Ismail N, Peters NC, Yurkowski DP, Bretscher PA. A valid ELISPOT assay for enumeration of ex vivo, antigen-specific, IFNgamma-producing T cells. J Immunol Methods. 1999. 227:99–107.

26. Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998. 188:409–414.

27. Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998. 16:1675–1682.

28. Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998. 8:177–187.

29. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002. 2:251–262.

30. Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001. 166:6099–6103.

31. Fu TM, Guan L, Friedman A, Schofield TL, Ulmer JB, Liu MA, Donnelly JJ. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J Immunol. 1999. 162:4163–4170.
32. Justewicz DM, Morin MJ, Robinson HL, Webster RG. Antibody-forming cell response to virus challenge in mice immunized with DNA encoding the influenza virus hemagglutinin. J Virol. 1995. 69:7712–7717.

33. Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol. 1997. 71:2715–2721.

34. Lee SW, Youn JW, Seong BL, Sung YC. IL-6 induces long-term protective immunity against a lethal challenge of influenza virus. Vaccine. 1999. 17:490–496.

35. Chen W, Antón LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000. 12:83–93.

36. Potter P, Tourdot S, Blanchard T, Smith GL, Gould KG. Differential processing and presentation of the H-2D(b)-restricted epitope from two different strains of influenza virus nucleoprotein. J Gen Virol. 2001. 82:1069–1074.

37. Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999. 17:51–88.

38. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002. 20:709–760.

39. Fomsgaard A, Nielsen HV, Bryder K, Nielsen C, Machuca R, Bruun L, Hansen J, Buns S. Improved humoral and cellular immune responses against the gp120 V3 loop of HIV-1 following genetic immunization with a chimeric DNA vaccine encoding the V3 inserted into the hepatitis B surface antigen. Scand J Immunol. 1998. 47(4):289–295.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download