INTRODUCTION
Toll-like receptors (TLRs) are primary sensor molecules that play an integral role in innate immunity via their capacity to recognize pathogen-associated molecular patterns that allow the detection of infection and inflammation (1). TLR stimulation of dendritic cells (DCs) and macrophages promotes the production of pro-inflammatory cytokines and the up-regulation of MHC and co-stimulatory molecules, which leads to the induction of T cell-mediated adaptive immune responses (2).
Although much of our knowledge of TLR function in the immune system comes from the study of innate immune cells, these molecules also are expressed in T cells. Early studies of TLRs in T cells have been conducted with CD4 T cells. Naïve human CD4 T cells express TLR2 after activation by TCR stimulation and TLR2 functions as a co-stimulatory receptor. Moreover, TLR2 also participates in the generation and maintenance of CD4 T cell memory (3). TLR3 and TLR9 ligand directly deregulate Bcl-xL in CD4 T cells, thus promoting survival (4). CpG DNA-mediated co-stimulation in CD4 T cells proceeds through the MyD88-dependent PI-3 kinase signaling pathway (5). According to a recent study, TLR2 stimulation activates Th1 effector cells without TCR stimulation through the enhanced activation of MAPKs. In contrast, no TLR affects the function of Th2 effector cells (6).
Several studies have reported the co-stimulatory effects of TLR on CD8 T cells. TLR2 engagement on CD8 T cells decreases the activation threshold for co-stimulatory signals delivered by APC (7). Quigley et al. showed that direct TLR2-MyD88 signaling in CD8 T cells plays a critical role in clonal expansion and memory formation against vaccinia viral (VV) infection (8). It has been also reported that MyD88-dependent signals are critical for survival of Lymphocytic choriomeningitis virus (LCMV)-specific CD8 T cells and sustained accumulation for viral clearance (9). Furthermore, TLR2 engagement on cytotoxic T-lymphocytes (CTL) augments antitumor activity against established B16 melanoma tumors (10).
Certain co-stimulatory molecules on activated T cells are known to primarily be involved in either the CD4 or CD8 T cell subset. For example, 4-1BB is preferentially involved in CD8 T cell-mediated immune responses (11). In the present study, we compared the expression and function of TLR2 on CD4 versus (vs.) CD8 T cells, which have not been directly compared yet. However, we found that TLR2 co-stimulation is more responsible for CD8 T cells than for CD4 T cells.
MATERIALS AND METHODS
Mice
Female B6 (H-2b) and Balb/c (H-2d) mice were purchased from Orient Bio Inc. (Seoul, Korea). TLR2-/- (H-2b) mice were provided by S. Akira (Osaka University, Osaka, Japan). All mice were used for the experiments at the age of 8~10 weeks.
Antibodies and reagents
The following antibodies were purchased from e-Bioscience (San Diego, CA) for flow cytometry: FITC-conjugated anti-mouse CD3 (145-2C11), TLR2 (6C2), and H-2b (AF6-88.5); PE-conjugated anti-mouse CD4 (GK1.5), CD8 (53-6.7), TLR2 (6C2), Bcl-2 (3F11), Bcl-xL (7B2.5), and IFN-γ (XMG1.2); PE-Cy5-conjugated anti-mouse CD4 (GK1.5) and CD8 (53-6.7); purified anti-CD16/32 (2.4G2) and purified anti-TLR2 (T2.5). LLO91-99 pentamer was obtained from ProImmune (Oxford, UK). Purified anti-mouse CD3 (145.2C11) was obtained from BD Biosciences (San Jose, CA). Pam3CSK4 was purchased from Invivogen (Carlsbad, CA).
Cell preparation, culture, and in vitro proliferation assay
Naïve T cells were isolated from spleen and lymph nodes of B6 using anti-CD90 (Thy1.2) magnetic beads (Miltenyi Biotech, Auburn, CA) after depletion of CD25+ cells. The cells were >97% CD3 T cells with a naïve phenotype. To prepare the CD4 T and CD8 T cells, CD11c+ and CD25+ cells were first depleted using anti-CD11c and -CD25 magnetic beads to remove Treg and lymphoid dendritic cells and then the cells were isolated using anti-CD4 or -CD8 magnetic beads, respectively. The cells were >97% CD3+ CD4+ or CD8+ T cells with a naïve phenotype. 2×105 cells were stimulated with soluble anti-CD3 (0.5 µg/ml) or pulsed with Pam3CSK4 (2 µg/ml) or LPS (2 µg/ml) for 64 h at 37℃ in a 5% CO2 environment. Proliferation was measured in triplicate cultures by the incorporation of [3H]thymidine (1 µCi/well, Amersham Pharmacia) during the last 12 h of culture. The incorporation of [3H]thymidine was measured with a β-counter (Wallac, Torrance, CA). For blocking analysis, purified cells were pretreated with anti-TLR2 antibody (2 µg/ml, T2.5) before the stimulation.
In vivo generation of alloantigen activated T cells
Responder T cells were purified from the spleen and lymph nodes of B6 (H-2b) mice using the anti-CD90 microbead separation system (Miltenyi Biotec). Cells (1×107) were suspended in PBS and transferred into lethally irradiated (1,000 cGy) Balb/c (H-2d) recipients via tail vein. Recipient splenocytes were isolated at 4 days after transplant, and cells were identified as donor T cells with anti-H-2b and -CD4 or -CD8 mAb and analyzed by flow cytometry.
In vivo generation of Listeria-specific memory CD8 T cells
Balb/c mice were infected intravenously (i.v.) with 3000 colony-forming units (CFU) of live L. monocytogenes. On day 25, the mice were reinfected with 5000 CFU of live bacteria intraperitoneally (p. i.); 5 days later, LLO91-99-specific CD8 T cells were determined using LLO91-99 pentamer.
Flow cytometry
To measure the expression of TLR2, cells were first incubated with FcR blocker (2.4G2) to block nonspecific antibody binding and then stained with PE-anti-TLR2 and PE-Cy5-anti-CD4 or CD8 and analyzed on a FACSCalibur flow cytometer (BD Biosciences) using the CellQuest software. To measure cell proliferation, cultured cells were treated with BrdU (2 µg, Sigma) for 1 h and washed with PBS. The cells were fixed, permeabilized, treated with DNase I, and stained with FITC-anti-BrdU using a BrdU Flow kit according to the manufacturer's instructions. To analyze cell death, cells were stained with FITC-annexin V and 7-AAD. To stain Bcl-2 and Bcl-xL, the cells were fixed, permeabilized, and stained with PE-anti-Bcl-2 or -Bcl-xL mAb. To stain intracellular IFN-γ, cells were fixed, permeabilized using the Cytofix/Cytoperm kit (BD Bioscience) according to the manufacturer's instructions, and incubated with PE-anti-IFN-γ mAb.
RESULTS
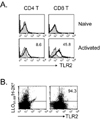
TLR2 expression is preferentially induced on CD8 T cells vs. CD4 T cells
To assess the expression pattern of TLR2 on CD4 vs. CD8 T cells, we performed flow cytometric analysis on naïve and alloantigen-activated T cells. TLR2 was not expressed on either naïve CD4 or CD8 T cells before adoptive transfer into allogeneic recipient. Four days after transfer, alloantigen-activated responder T cells induced TLR 2 expression. However, the expression levels were much higher on CD8 T cells (45.7±4.8%) than on CD4 T cells (5.4±3.2%) (Fig. 1A). Furthermore, Listeria-specific memory CD8 T cells constitutively expressed TLR2 (Fig. 1B). These data indicate that TLR2 is preferentially expressed on CD8 T cells following activation.
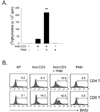
TLR2 co-stimulation dominantly enhances CD8 T cell expansion more than CD4 T cell expansion
To test the effect of TLR2 co-stimulation on the proliferation of total T cells or CD4 vs. CD8 T cells, T cells were isolated as described in Materials and Methods and incubated with soluble anti-CD3 (anti-CD3s) in the presence or absence of TLR2 ligand Pam3CSK4. First, we observed that the co-stimulation of total T cells with Pam3CSK4 led to a seven-fold enhancement of anti-CD3-induced proliferation (Fig. 2A). We next evaluated the ratio of CD4 vs. CD8 T cells in the proliferative capacity of the total T cell population that was enhanced by TLR2 co-stimulation, and performed the BrdU incorporation assay. Fig. 2B shows that there were more CD8 T cells than CD4 T cells in the increased proliferative capacity of total T cells. To further confirm the direct effect of TLR2 signaling on T cell subsets, we isolated highly purified populations of naïve CD4 or CD8 T cells after the depletion of CD11c+ and CD25+ cells to remove contaminating lymphoid DCs and natural Treg, respectively (>97% purity). T cell subsets were then incubated with anti-CD3s in the presence or absence of Pam3CSK4. The co-stimulation of CD8 T cells with Pam3CSK4 led to a 11-fold enhancement of anti-CD3-induced proliferation. However, the CD4 cell proliferation was increased five-fold (Fig. 3). Taken together, these results indicate that TLR2 co-stimulation is preferentially involved in CD8 T cell expansion rather than CD4 T cell expansion.
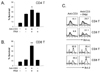
TLR2 co-stimulation elevates CD8 T cell survival more strongly than CD4 T cell survival
To further assess the effect of TLR2 co-stimulation on the survival of CD4 vs. CD8 T cells, isolated naïve CD4 and CD8 T cells were stimulated with anti-CD3s in the presence or absence of Pam3CSK4. Survival was then detected by annexin V plus 7-AAD staining at 64 h following activation. Pam3CSK4 increased the activated CD8 T cell survival from 12% to 40% (Fig. 4B). However, the CD4 T cell survival was increased from 25% to 35% by TLR2 co-stimulation (Fig. 4A). Members of the Bcl family are reported to be key mediators of activated T cell survival following TLR2 co-stimulation (4). Therefore, we compared the levels of these molecules following TLR2 ligand treatment of CD4 or CD8 T cells. We observed more significant increases in Bcl-xL protein in Pam3CSK4-treated CD8 T cells than in CD4 T cells. However, Bcl-2 protein levels were similar in CD4 and CD8 T cells (Fig. 4C). These data indicate that TLR2 co-stimulation is preferentially involved in CD8 T cell survival versus that in CD4 T cells. Thus, it is associated with specific Bcl-xL up-regulation.
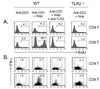
Specificity of TLR2 co-stimulation
To exclude the possibility that the effects caused by Pam3CSK4 were non-specific, we tested the assay using anti-TLR2 monoclonal antibody, which was added to the culture before the stimulation. As shown in Fig. 5, the enhanced proliferation with Pam3CSK4 was completely reversed in both CD4 and CD8 T cells by anti-TLR2 treatment as was IFN-γ production. In addition, T cells purified from TLR2-/- mice exhibited no response to Pam3CSK4 in terms of either the proliferation (Fig. 5A) or the IFN-γ production (Fig. 5B), indicating that Pam3CSK4 acts through TLR2-dependent signaling pathways.
DISCUSSION
TLR in T cells can function as a co-stimulatory molecule for both CD4 and CD8 T cell activation (12). In this study, we have confirmed that the TLR2 ligand Pam3CSK4 provides a direct potent co-stimulatory effect on TCR-mediated T cell proliferation. However, we found that TLR2 co-stimulation was biased toward CD8 T cells rather than CD4 T cells. For instance, the addition of Pam3CSK4 increased the anti-CD3-mediated proliferation of total T cells by 7-fold (Fig. 2A). In this increased proliferative capacity, CD8 T cells were found in a higher proliferative ratio than CD4 T cells (Fig. 2B), which was confirmed on an isolated subset of T cells (Fig. 3). We also observed that TLR2 co-stimulation promoted the survival of CD8 T cells more than that of CD4 T cells (Fig. 4A, B). This was caused not by Bcl-2 but by increased Bcl-xL (Fig. 4C). In fact, the different sensitivity to TLR2 co-stimulation is probably related to the expression levels on CD4 versus CD8 T cells. The surface expression was more highly induced following activation on CD8 T cells compared with that on CD4 T cells (Fig. 1A). Taken together, these results indicate that CD8 T cells preferentially respond to TLR2 co-stimulation.
CD8 T cells are critical for prevention of acute and chronic viral infections (13) as well as for tumor eradication (14). In recent studies, the physiological significance of TLR2 on CD8 T cell-mediated effector immune responses has been reported. Quigley et al. reported that TLR2-/- and MyD88-/- CD8 T cells had severely diminished clonal expansion in response to vaccinia viral (VV) infection, which involved the TLR2 co-stimulation on VV-specific CD8 T cells (8). The study also reported that long-lived memory CD8 T cells could not develop in the absence of direct TLR2-MyD88 signaling. We also observed that TLR2 is constitutively expressed in Listeria-specific memory CD8 T cells (Fig. 1B). Indeed, rapid Listeria-specific memory CD8 T cell formation is affected by primary infection (15). It may be related to TLR2 expression that is induced on Listeria-specific CD8 T cells during the primary infection time. The TLR2 expression may affect rapid expansion of the memory CD8 T cells during the secondary infection period.
Our data also indicated that TLR2 co-stimulation decreased the threshold for antigen-specific signaling through TCR. We stimulated T cells with soluble anti-CD3 to provide weak TCR-mediated activation. Although, under these conditions, TLR2 signaling effectively elicited the expansion and IFN-γ production of CD8 T cells (Fig. 5), it can be speculated that TLR2 signaling affects autoreactive CD8 T cell responses. Autoreactive T cells recognize autoantigens, which are basically presented by immature DCs that give feeble TCR signaling, resulting in ignorance or anergy (16). Under pathogen infection conditions, TLR2 signaling enhances the direct pathway of autoreactive T cell activation by co-stimulation as well as the indirect pathway by induction of DC maturation. A number of animal models for autoimmune disease probably involve TLR signaling in their pathogenesis (17,18). Our data indicate that promoting the expansion and the effector function of CD8 T cells by TLR2 signaling was completely reversed by the anti-TLR2 mAb, T2.5 (Fig. 5), the therapeutic activity of which has been reported in the sepsis model (19). Therefore, T2.5 might be become a valuable therapeutic agent for CD8 T cell-mediated pathological conditions in the presence of TLR ligand.
Although it has been recently suggested that TLR2 could be particular in its ability to co-stimulate CD4 and CD8 T cells, in this present study, we find that its dominant effect appears to be the regulation of CD8 T cell activation. These observations suggest a potential therapeutic role for this molecule in the management of cancer and chronic infectious diseases as well as autoimmune diseases.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download