Abstract
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory disorder with an unknown etiology. IBD is composed of two different disease entities: Crohn's disease (CD) and ulcerative colitis (UC). IBD has been thought to be idiopathic but has two main attributable causes that include genetic and environmental factors. The gastrointestinal tract in which this disease occurs is central to the immune system, and the innate and the adaptive immune systems are balanced in complex interactions with intestinal microbes under homeostatic conditions. However, in IBD, this homeostasis is disrupted and uncontrolled intestinal inflammation is perpetuated. Recently, the pathogenesis of IBD has become better understood owing to advances in genetic and immunologic technology. Moreover, new therapeutic strategies are now being implemented that accurately target the pathogenesis of IBD. Beyond conventional immunesuppressive therapy, the development of biological agents that target specific disease mechanisms has resulted in more frequent and deeper remission in IBD patients, with mucosal healing as a treatment goal of therapy. Future novel biologics should overcome the limitations of current therapies and ensure that individual patients can be treated with optimal drugs that are safe and precisely target IBD.
The entire surface of the human intestine reaches 200~400 m2 (1). Moreover, it occupies a central position as the frontier of the innate immune system. The inner cell lining of the intestine works not only as a barrier to protect the host from harmful pathogens but also as a place where interactions with commensal microorganisms occur. These interactions are delicately modulated by the intestinal immune system and contribute to immune homeostasis. For various reasons, idiopathic intestinal inflammations such as inflammatory bowel disease (IBD) can occur when this homeostasis is disrupted (23).
IBD is a multifactorial immune disorder characterized by chronic relapsing inflammation of the intestine (4). It is classified into two different disorders: Crohn's disease (CD) and ulcerative colitis (UC). Clinically, CD and UC share similar symptoms, including diarrhea, hematochezia, and abdominal pain, whereas the location and depth of inflammation, as well as complications and prevalence can differ. Currently, the exact etiology of IBD is unclear. However, it is believed that disturbance of the immune system and/or imbalanced interactions with microbes leads to development of chronic intestinal inflammation when certain environmental factors trigger genetically susceptible hosts. Traditionally, Th1 cells have been thought to play an important role in pathogenesis related to the chronicity of intestinal inflammation, especially in CD, whereas Th2 cells have been thought to play an important role in UC (5). Recently, however, activation of Th17 cells and imbalance of Th17/regulatory T (Treg) cells are recognized to be an important component in the development of intestinal inflammation (6). Since tumor necrosis factor (TNF)-α has been identified as a key cytokine in IBD pathogenesis, the introduction of anti-TNF-α treatment has led to the development of disease-modifying drugs (789). Compared to conventional therapies, anti-TNF agents have higher rates of remission induction and maintenance. Moreover, these drugs have been able to obtain mucosal healing through targeted immune suppression. However, about a third of patients with IBD still do not show an appropriate response to existing therapies. This high rate of treatment failure suggests that there are still unknown aspects regarding the mechanism of IBD. Encouragingly, however, dozens of novel agents based on recent advances in our understanding of the mucosal immune system for IBD pathogenesis have been developed and are now in clinical trials worldwide. In this review, we will describe our current knowledge of the mucosal immune system in terms of IBD pathogenesis and discuss its therapeutic implications.
The intestinal epithelial cell (IEC) layer consists of several different cells, including enterocytes, goblet cells, neuroendocrine cells, Paneth cells, M cells, and epithelial resident intestinal stem cells. These cells structurally constitute crypts and villi, with a single columnar cell lining with a tight junction, and secrete mucus containing anti-microbial peptides; these cells separate intra-luminal pathogens from the subepithelial lamina propria (31011).
To protect mucosa, a mucus layer covers the outer epithelial surface. The mucus layer is composed of glycosylated mucin from goblet cells as well as defensins from Paneth cells and IECs. A major component of mucin is encoded by Muc2, and spontaneous colitis develops upon deletion of Muc2 in mice (12). A study showed that aberrant mucin production was accompanied by endoplasmic reticulum (ER) stress (13). Goblet cell depletion and a reduced mucus layer are characteristic findings in patients with UC (14). In addition to mucin, Paneth cells secret α-defensin, whereas most IECs produce β-defensin. Paneth cells are known to play an important role in the homeostasis of the intestinal epithelium. Genetic alterations or ER stress causing Paneth cell dysfunction or depletion result in dysbiosis of commensal flora and increased susceptibility to intestinal inflammation (15). It is known that IBD patients often have this Paneth cell dysfunction (16). Paneth cell abnormalities are thought to be a very early event in IBD development, particularly in CD. Therefore, there are studies examining the effects of applying ER-stress-reducing methods to IBD treatment. A study showed that the chemical chaperones tauroursodeoxycholate (TUDCA) and 4-phenylbutyrate (PBA)—small molecules that can reduce ER stress by facilitating protein folding—prevented the induction of intestinal inflammation in mice (17).
Epithelial integrity is maintained by tight junctions between IECs. When the permeability of the intestinal epithelium is increased, external pathogens are easily introduced, which is known to affect the pathogenesis of IBD (18). Several lines of evidence showed that single-nucleotide polymorphisms in the organic cation transporter (OCTN), which mediates the transport of organic cations across the cell membrane, were associated with CD susceptibility (1920). IECs also play a role as communicator between pathogens and lamina propria. Only small amounts of bacteria are generally capable of moving into the intestinal epithelium. This translocation is a method of antigen sampling and immune surveillance for the intestinal mucosal immune system that is essential for the host's immune homeostasis (21). However, when the integrity of the intestinal epithelial layer is broken, a high influx of intestinal contents and/or a high burden of microorganisms is thought to initiate and maintain a sustained inflammatory response, which is considered to be one of the mechanisms underlying IBD (22). For example, in an animal model in which the barrier function of the intestinal epithelial layer is reduced, such as in mice with a dominant negative N-cadherin mutation (23) or mice lacking NOD1 and NOD2 (24), the mice develop IBD-like enteritis. Moreover, several genetic studies have identified several candidate genes in patients with UC (2526), such as CDH1 and LAMB1, which are involved in regulation of the epithelial barrier. Therapeutic attempts to restore mucosal barrier function have also been attempted. Phosphatidylcholine (lecithin) is abundant in the mucus of healthy colons, whereas reduced lecithin levels were observed in UC patients (27). A phase IIA, double-blind, randomized, placebo-controlled study showed that oral administration of lecithin was effective for achieving clinical remission in patients with chronic active UC (28).
Generally, there are approximately 1011~1014 enteric commensal microorganisms from 300~500 bacterial species (2930). Under normal circumstances, most commensal bacteria play an essential role in protecting intestinal homeostasis. They affect crucial nutrient provision, development of the immune system, and modulation of energy metabolism (531). The majority of commensal bacteria consist of gram-negative bacteria, such as Bacteroidetes, and gram-positive bacteria, such as Firmicutes (32). Other minor divisions are comprised of Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia (32). Those mucosa-associated phyla are reduced in diversity and amount in patients with IBD compared to that in healthy humans (53334). How ever, commensal microorganisms can be noxious for intestinal inflammation under certain circumstances (35). There are some clues that commensal bacteria play an important role in the development of IBD. First, empiric antibiotic treatment has been effective in some IBD patients (36). Second, IBD patients have increased titers against indigenous bacteria (37). Third, genetic variants that are associated with bacterial detection, such as NOD2 (38), and T cell immunity, such as IL23R (39), are implicated in IBD. Fourth, most animal models of colitis require commensal bacteria for the initiation of intestinal inflammation (40). In addition, recent studies have focused on the contribution of other enteric microorganisms, such as viruses or fungi, for IBD development. For example, a study (41) revealed that altered amounts and compositions of enteric virus were related to experimental colitis. In particular, mice without Toll-like receptor (TLR) 3 and TLR7 were more susceptible to the induction of colitis. Likewise, Iliev et al. reported that mice deficient for Dectin-1, which is an innate immune receptor responsible for interacting with commensal fungi, showed increased susceptibility to colitis (42).
To date, several pathogens have been proposed as causative microorganisms for IBD development. Recent studies showed Proteobacteria, especially adherent-invasive Escherichia coli (AIEC), as one of the candidates. AIEC has been detected more frequently in patients with CD than in healthy subjects (434445). AIEC is known to be able to invade epithelium and replicate within macrophages (46). Some investigators isolated AIEC from the ileum of patients with CD (4748). However, AIEC was rarely found in the colon tissues of CD patients and was not identified in UC patients (49), suggesting that AIEC performs an important role in the occurrence of small bowel inflammation (18).
In contrast, Clostridium cluster XIVa and IV are thought to be a crucial part of gut homeostasis through Treg cell accumulation (50). Foxp3+CD4+ Tregs are known to be abundant in the colonic lamina propria and are the most important immune-regulating cells (51). Several studies showed that Treg cells were strongly affected by intestinal microbiota (52). In particular, Treg cells stimulated by CBir1, a microbiota flagellin, induce IgA+B cells in the intestine. As a result, decreased pathogenic loading by IgA leads to down-regulation of systemic T cell activation (53). An experimental murine model with an increased Clostridium XIVa/IV population was resistant to allergy and intestinal inflammation (50). Conversely, patients with IBD showed a reduced Clostridium XIVa/IV compared to that in controls (345455).
Observations of dysbiosis in IBD patients led to efforts to restore microbiota to a normal composition. Fecal microbial transplantation (FMT) has emerged as a novel treatment in patients with IBD. One randomized control trial involving 75 UC patients showed a significantly higher remission rate (24%) in patients receiving FMT from unrelated donor enemas than that in the placebo group (5%) (56). However, a second randomized control trial with 48 UC patients reported a negative result (57). Currently, there are no randomized control trials comparing FMT with placebo treatment in CD patients. A meta-analysis using four case series data in 38 CD patients revealed a 60.5% pooled response rate (58). However, their outcome was not that of mucosal remission but of clinical response. Therefore, the effectiveness of FMT as a therapeutic application for IBD remains unclear. Furthermore, optimal donor selection, delivery methods, and donor feces processing have not yet been standardized. Probiotics are nutritional supplements that contain microorganisms that benefit the host's health when administered in the proper amount. Attempts have also been made to treat IBD by improving intestinal microbial balance through probiotics. In an experimental colitis model, probiotics showed an anti-inflammatory effect through TLR9 signaling (59). A recent meta-analysis using 23 randomized controlled trials showed that administration of probiotics was associated with benefits regarding induction and maintenance of remission in patients with UC but not in CD (60). Further studies are warranted to draw a concrete conclusion in terms of the therapeutic effects of probiotics in IBD.
The innate immune system is at the forefront of defending against external pathogens in the human immune system. The innate immune system provides rapid and nonspecific protection to the host through pattern-recognition of pathogens, whereas the adaptive immune system mediates highly selective and long-lasting immunity. The innate immune system of the intestine is composed of intestine epithelia, macrophages, monocytes, neutrophils, eosinophils, basophils, dendritic cells (DCs), and natural killer cells. Intraluminal pathogens continuously communicate with innate immune cells through diverse innate immune receptors such as TLRs, NOD, leucinerich repeat receptors (NLRs), C-type lectin receptors (CLRs), and retinoic acid-inducible gene 1-like receptors (RLRs) (61). When intestinal macrophages and DCs sense pathogen-associated molecular patterns (PAMPs) of microbes, activated signal pathways, such as NF-κB, produce proinflammatory cytokines, chemokines, and anti-microbial peptides (62). Activation of macrophages by these cytokines and chemokines plays a role in the direct elimination of pathogens through free radicals and proteases and also results in antigen presentation to the adaptive immune system. Antigen presenting cells (APCs), such as DCs and macrophages, have a key role in connecting the innate and adaptive immune system. Comparing that macrophages perform antigen presentation and have a phagocytic function, activated DCs present intraluminal pathogens to naïve CD4+ T cells at secondary lymphoid organs of the gut and modulate the polarization of naïve CD4+ T cells to Treg cells and helper T cells, including Th1, Th2, and Th17 cells.
Under non-inflammatory conditions, TLR signaling leads to tolerance towards luminal pathogens through down-regulation of pattern-recognition receptors and promotes mucosal wound healing. In IBD patients, impaired TLR signaling often leads to increased intestinal permeability and inappropriate mucosal healing. For example, TLR2-deficient mice showed an increased mortality rate after damage was induced to the colon mucosa via chemicals. TLR2 signaling stimulates the production of trefoil factor (TFF) 3 and restores damaged mucosa. In mice lacking TLR2, mortality was reduced when TFF3 was administered (63). Likewise, genetic studies showed the association between CD and the nucleotide oligomerization domain (NOD) 2 gene. NOD2 polymorphisms lead to an impaired response to bacterial peptidoglycan sensing. While the exact mechanism between CD and impaired NOD2 function is still unclear, NOD2-mediated chronic stimulation is thought to be one of the factors controlling proinflammatory cytokine production. Recent genome-wide association studies (GWAS) showed relationships between a variety of single-nucleotide polymorphisms (SNPs) and IBD risks: microbial sensing (NOD2, IRF5, NFKB1, RELA, REL, RIPK2, CARD9, and PTPN22), microbial elimination (ATG16L1, IRGM, and NCF4), and integration of antimicrobial adaptive immune responses (IL23R, IL10, IL12, IL18RAP/IL1R1, IFNGR/IFNAR1, JAK2, STAT3, and TYK2) (646566). Recent studies have shown that autophagy plays an important role in the innate immune system. Autophagy is a biological process that activates cellular autodigestion of the cell's own cytosolic materials, including intracellular microbes. Additionally, autophagy enables antigen presentation by major histocompatibility complex class II. Repeated GWAS studies consistently showed the association between CD and autophagy-related genetic polymorphisms, such as ATG16L1 and IRGM (6768697071). The accumulation of both macrophages and DCs is observed in the lamina propria of IBD patients and in experimental colitis models (3). If interactions between DCs and T cells are interrupted, experimental T cell-mediated colitis is prevented (72). Moreover, DCs and macrophages also play a role in maintaining gut homeostasis against the inflammatory conditions of the intestine. A study showed that a pro-resolution mediator, prostaglandin D (2), was specifically up-regulated in UC patients with long-term remission (73). Likewise, another study revealed that a SNP associated with low levels of CD39/ENTPD1, which hydrolyzes proinflammatory nucleotides and generates adenosine, was related to an increased risk of developing CD (74). Taken together, an impaired innate immune response might promote IBD development via inappropriate stimulation of adaptive immunity through failure to control microorganisms (66). Therefore, researchers have focused on enhancing innate immunity as a therapeutic target of IBD. For example, certain growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), which are critical for modulation of cellular proliferation, differentiation, angiogenesis, and inflammation, have been evaluated for treatment of intestinal inflammation (75).
Chronic inappropriate activation of the adaptive immune system against commensal microorganism has been thought to be the main pathogenesis of IBD. Increased production of IFN-γ from Th1 cells and cytokines related with Th17 cell, such as IL-17A/F, IL-21, IL-22, and CXCL8, are observed in the intestine of CD patients, while T cells from the lamina propria of UC patients highly produce Th2 cell-related cytokines, such as IL-5 and IL-13 (37677).
Classically, immune-modulating treatments of IBD have focused on adaptive immunity. Corticosteroids have been widely used to treat acute flares of IBD since Truelove and Witts reported the effectiveness of oral corticosteroids in patients with UC in 1955 (78). Suppression of proinflammatory cytokines, such as TNF-α and IL-1β, is known to be the primary mechanism underlying how corticosteroids control IBD (79). In addition, recent studies highlighted that corticosteroids play an important role in regulation of T helper cell differentiation and type I interferon (IFN) production (79). Clinically, corticosteroids are effective for remission induction of IBD. A study observed that the first course of oral corticosteroid treatment achieved 89.5% of therapeutic response after 1 month, 69.5% after 4 months, and 56.6% after 1 year in patients with CD (80). Likewise, various immunomodulators that down-regulate the proinflammatory cytokines of T cells have been a well-established treatment for IBD. For example, cyclosporine A and tacrolimus are used for remission induction of active UC, and methotrexate is used for chronically active CD (818283). Of these treatments, the most widely used agent is thiopurines, such as azathioprine and 6-mercaptopurine. Thiopurine inhibits purine nucleotide synthesis and breaking of DNA in leukocytes via 6-thioguanine nucleotides (6-TGNs), which is the effector product of thiopurine metabolism (84). Moreover, thiopurine sup presses CD4+ T cell acti vity and promotes T cell apoptosis by inhibiting GTPase Rac1 in inflamed intestine (85). Recently, a study showed that autophagy-relate genetic variant, ATG16L1, was associated with the good clinical towards thiopurine treatment in patients with CD but not in UC (86). In addition, a study showed that local administration of thioguanine improved murine colitis by promoting autophagy and killing translocated bacteria at the site of the inflamed intestine independently of systemic myelosuppression (87). Therefore, thiopurine probably works by multiple mechanisms to improve IBD. Meanwhile, thiopurine may cause life-threatening leukopenia. The TPMT mutation is known to be associated with this complication (88). More specifically, a study involving immunochip genotyping of Asians revealed that NUDT15 SNP was strongly related to thiopurine-induced leukopenia, and another study showed an association between FTO variant and leukopenia by GWAS analysis (8990). Although nonspecific immunosuppression using immunomodulators is generally safe and effective for disease control to an extent, advances in the understanding of the specific mechanisms of IBD led to the development of targeted treatment, i.e., biologics.
The era of biologic therapy began with an anti-TNF agent, infliximab, in patients with CD (91). TNF-α is a proinflammatory cytokine that is produced by activated macrophages, monocytes, and T lymphocytes (7). Intestinal specimens of CD patients were shown to have increased levels of TNF-α protein and mRNA expression (92). Excessive production of TNF-α using experimental deletion of the adenosine-uracil (AU)-rich elements (ARE) from the 38-untranslated region (38-UTR) of the TNF-α gene in mice resulted in development of chronic inflammatory arthritis and CD-like IBD phenotype (93). Another experimental study revealed that inhibiting TNF was able to improve dextran sulfate sodium (DSS)-induced colitis in a mice model (94). Therefore, TNF-α has been thought to play a pivotal role in the development of IBD.
TNF-α has two forms in the human intestine: transmembrane TNF (mTNF) and soluble TNF (sTNF). mTNF is generally expressed on the surface of CD14+ macrophages and targets TNF-R2 of T cells, whereas sTNF is secreted by several immune cells as a signaling molecule and targets TNF-R1 of effector cells (9596). In IBD, increased levels of both mTNF and sTNF play various pro-inflammatory functions in the inflamed gut, such as angiogenesis, Paneth cell death, matrix metalloproteinase production from myofibroblasts, and the undermining of the barrier function of IECs (77). Recent studies have shown that interaction between mTNF and TNF-R2 is more important for IBD pathogenesis than that of sTNF and TNF-R1 (9798).
Several anti-TNF-α monoclonal antibodies have been developed since infliximab (chimeric antibody with a murine sequence) and adalimumab (fully humanized antibody) showed effectiveness for induction and maintenance of remission, as well as mucosal healing of IBD (99100101102103). A humanized, pegylated anti-TNF Fab fragment, certolizumab pegol, also showed benefits and was approved for CD treatment (104). Moreover, golimumab, a transgenic fully human monoclonal immunoglobulin G1 antibody, was recently launched for the treatment of UC (105). However, another anti-TNF agent that targets sTNF, etanercept, showed no benefits regarding treatment of IBD (106107). Although there are several limitations of anti-TNF treatment, such as safety issues, relatively high cost, and loss of effectiveness, the potential benefits of anti-TNF agent may outweigh these drawbacks, because blocking the TNF signal in IBD works through various mechanisms, including T cell apoptosis, inhibiting T cell differentiation, induction of Treg cells and macrophages, and barrier improvement (96108109110). Therefore, efforts to overcome the drawbacks of anti-TNF agents, such as oral formulations, bacteria-producing nanobodies, and therapeutic vaccines against TNF are still in development (111112113).
Other important cytokines in the treatment of IBD are related to Th17 cells (IL-17A, IL21, IL-22, and IL-23) (114). Th17 cells are differentiated from naïve CD4+ T cells that are stimulated with transforming growth factor (TGF)-β and IL-6 in mice models. The inflamed intestinal tissue of IBD patients was shown to contain higher levels of Th17 cells and its cytokines (115). Additionally, a chemoattractant of Th17 cells, CCL20, is also elevated in the intestinal mucosal of IBD patients (116). While the exact role of Th17 cells and their cytokines in regards to intestinal inflammation has not yet been fully understood, the balance between Th17 and Treg cells are thought to be an important aspect in development of IBD (117). Based on those viewpoints, a humanized IFN-γ antibody, fontolizumab, was developed. However, it did not show a satisfactory result in patients with moderately to severely active CD (118). Likewise, an attempt to target IL-17A through a monoclonal antibody, secukinumab, in patients with CD also failed (119). In experimental colitis models, Th17 cells and their related cytokines are thought to play both inflammatory and anti-inflammatory roles in the intestine (114). Anti-inflammatory cytokines such as IL-22 are also produced by Th17 cells. These cytokines are known to promote epithelial proliferation, mucosal healing, and anti-microbial peptides in the mucus (120). Moreover, plasticity reflecting the environmental conditions during the inflammatory process between Th1/Treg and Th17 cells is observed, and these reciprocal alterations are thought to be important for maintaining intestinal homeostasis (121). The contribution of Th17 cells for intestinal inflammation might be controlled by more detailed interactions between immune cells.
Considering the complex interactions between various cytokines that contribute IBD, the targeting of multiple cytokines is thought to be a reasonable approach in the treatment of IBD. Ustekinumab, a human monoclonal antibody against the p40 subunit that is a component of both IL-12 and IL-23, is theoretically relevant for the treatment of CD involving both Th1 and Th17 aspects of CD. IL-12 induces Th1 polarization of naïve CD4+ T cells and IL-23 promotes Th17 cell differentiation (122). In moderate-to-severe CD patients, ustekinumab showed a significant clinical benefit in both remission induction and maintenance (123). Similarly, other biologics targeting the IL-12/23 pathway, such as ABT-874 (124) and apilimod mesylate (125), are under evaluation. IL-10-deficient mice can develop spontaneous T cell-dependent colitis and colitic cancer (126127). IL-10 is known as an anti-inflammatory cytokine and the UC-related IL10 gene variation was also noted from a GWAS study. Given this, a study used an interesting approach to treat experimental murine colitis involving the genetically engineered IL-10-secreting bacteria Lactococcus lactis. Intra-gastric administration of this bacteria resulted in a 50% reduction of DSS-induced colitis in IL-10-knockout mice (128).
Interactions between proinflammatory cytokines and their receptors lead to activation of intracellular signal transduction and production of inflammatory proteins. Janus kinase (JAK)-signaling transducers and activator of transcription (STAT) cytokine signaling pathways are recently thought to be a potential therapeutic target of IBD. Because various key cytokines such as IFN-γ, IL-2, IL-4, IL-7, IL-9, IL-15, IL-12, IL-21, IL-22, and IL-23 depend on the JAK-signaling pathway, inhibiting JAK might result in the downregulation of multiple inflammatory cytokines (129). The JAK family consists of four intracellular proteins, JAK1, JAK2, JAK3, and tyrosine kinase (TYK) 2 (130). A JAK1/JAK3 inhibitory small molecule, tofacitinib, showed promising results for the treatment of UC in a phase II study. In 194 moderately to severely active UC patients, 78% of patients who received 15 mg of oral tofacitinib twice a day showed a favorable clinical response (131). However, these clinical responses were not repeated in patients with moderate-to-severe CD in another phase II trial (132). Currently, various kinds of JAK inhibitors are now in development and awaiting clinical results.
In IBD patients, defective tumor necrosis factor (TGF)-β1 activity is related to up-regulation of SMAD7. Inhibition of TGF-β1 in healthy human intestines results in increased production of proinflammatory cytokines (133). In a double-blind, placebo-controlled, phase 2 trial, an oral SMAD7 anti-sense oligonucleotide, mongersen, showed a better clinical remission rate in patients with CD compared to that in patients who received a placebo (134).
Several approaches involving the targeting of differentiation and activation of T cells have been attempted. However, a cytotoxic T-lymphocyte antigen 4 (CTLA4) agonist, abatacept, which blocks APC and T cell interaction, showed no clinical benefits in regards to either remission induction or maintenance in patients with IBD (135). Similarly, a humanized monoclonal antibody to CD3 on the activated T cell, visilizumab, was shown to have no clinical benefits for treatment of IBD in phase III study (91).
Effector lymphocytes must travel from the periphery to the intestine for development of IBD. In this process, various adhesion molecules act on specific lymphocytes. Different lymphocytes express specific cell surface adhesion molecules targeting specific organs. Therefore, selective inhibition of those adhesion molecules potentially has a therapeutic role for IBD. Natalizumab is a humanized monoclonal antibody that binds the α4 subunit of integrin on T cells. Theoretically, gut homing of T cell results from the interaction between α4β7 integrin and mucosal vascular addressing cell adhesion molecule 1 (MAdCAM-1). Natalizumab showed clinical benefits for remission maintenance (136). However, natalizumab also blocks α4β1 integrin, which is important for T cell homing to the central nervous system, and fatal complications such as progressive multifocal leukoencephalopathy have emerged as a result (137). Therefore, using natalizumab for IBD treatment is restricted in some countries such as the USA. A more recently developed intestine-specific, anti-adhesion molecule, vedolizumab (monoclonal antibody against α4β7), showed promising results for inducing and maintaining remission in UC, and clinical remission in CD, with a relatively good safety profile (138139). Etrolizumab, a monoclonal antibody against the β7 subunit of integrin, is under phase III studies for CD and UC. Etrolizumab acts as dual inhibitor of the α4β7-MAdCAM-1 and αEβ7-E-cadherin interactions. Therefore, etrolizumab prevents both gut homing of lymphocytes and intraepithelial leukocyte retention of intestinal mucosa. A recent phase II trial in UC patients treated with etrolizumab showed a significantly higher clinical remission rate than that in the placebo group (140). Direct inhibition of MAdCAM-1 by its monoclonal antibody, PF-00547659, was also developed and attempted in clinical trials. Recently, mixed results were obtained, with one study showing significantly higher clinical remission rate in UC (TURANDOT study) compared to that in the placebo group, whereas another study yielded negative results for CD (OPERA study) (141142).
As IBD-related research progresses, understanding of these diseases is deepening (Fig. 1). However, it is not believed that only one obvious mechanism of disease will be readily apparent. With the opening of the era of biologics, it has become possible to expect deep remission in IBD patients, unlike in the past; however, about one-third of patients still do not show clinical improvement to the biological agents. A variety of new biologics specific to IBD pathogenesis are now emerging and under clinical investigation (Fig. 2). With this development, more and more patients will benefit from these novel agents. Moreover, future IBD therapy should be approached in terms of “patient-customized treatment,” and it is anticipated that it will be a great help in clinical practice to have a drug repertoire targeting various mechanisms of the disease.
Figures and Tables
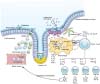 | Figure 1Intestinal immune system. IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; TGF, transforming growth factor; Th, helper T cell; Treg, regulatory T cell; TCR, T cell receptor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cell; TLR, toll-like receptor; NOD, nucleotide oligomerization domain. |
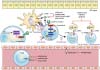 | Figure 2Biologics regarding therapeutic targets (black: showed benefits; violet: no benefits). APC, antigen presenting cell; IEC, intestinal epithelial cell; TNF, tumor necrosis factor; MHC, major histocompatibility complex; TCR, T cell receptor; JAK, Janus kinase; TGF, transforming growth factor; IL, interleukin; MAdCAM, mucosal vascular addressing cell adhesion molecule. |
ACKNOWLEDGEMENTS
This study was funded by the grant 15182MFDS507 from the Ministry of Food and Drug Safety, Korea.
References
1. Schulenburg H, Kurz CL, Ewbank JJ. Evolution of the innate immune system: the worm perspective. Immunol Rev. 2004; 198:36–58.

2. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011; 474:298–306.

3. Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013; 62:1653–1664.

6. Cheon JH. Genetics of inflammatory bowel diseases: a comparison between Western and Eastern perspectives. J Gastroenterol Hepatol. 2013; 28:220–226.

7. Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000; 51:289–298.

8. Cheon JH. Understanding the complications of anti-TNF therapy in East Asian patients with inflammatory bowel disease. J Gastroenterol Hepatol. DOI: 10.1111/jgh.13612.
9. Choi CH, Song ID, Kim YH, Koo JS, Kim YS, Kim JS, Kim N, Kim ES, Kim JH, Kim JW, Kim TO, Kim HS, Kim HJ, Park YS, Park DI, Park SJ, Song HJ, Shin SJ, Yang SK, Ye BD, Lee KM, Lee BI, Lee SY, Lee CK, Im JP, Jang BI, Jeon TJ, Cho YK, Chang SK, Jeon SR, Jung SA, Jeen YT, Cha JM, Han DS, Kim WH. Efficacy and safety of infliximab therapy and predictors of response in Korean patients with Crohn's disease: A nationwide, multicenter study. Yonsei Med J. 2016; 57:1376–1385.

10. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009; 71:241–260.

11. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014; 13:3–10.

12. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006; 131:117–129.

13. Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008; 5:e54.

14. Jass JR, Walsh MD. Altered mucin expression in the gastrointestinal tract: a review. J Cell Mol Med. 2001; 5:327–351.

15. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010; 28:573–621.

16. Kaser A, Blumberg RS. Endoplasmic reticulum stress and intestinal inflammation. Mucosal Immunol. 2010; 3:11–16.

17. Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013; 144:989–1000.

18. Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014; 20:6–21.

19. Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G, Griffiths AM, St George-Hyslop PH, Siminovitch KA. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004; 36:471–475.

20. Park HJ, Jung ES, Kong KA, Park EM, Cheon JH, Choi JH. Identification of OCTN2 variants and their association with phenotypes of Crohn's disease in a Korean population. Sci Rep. 2016; 6:22887.

21. Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009; 325:617–620.

22. Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006; 3:390–407.

23. Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995; 270:1203–1207.

24. Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD, Verdu EF. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1-/-; Nod2-/- mice. Inflamm Bowel Dis. 2012; 18:1434–1446.

25. Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG, Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, McCarthy MI, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CC, Barrett JC, Bellenguez C, Davison D, Freeman C, Strange A, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Deloukas P, Peltonen L, Mathew CG, Blackwell JM, Brown MA, Corvin A, McCarthy MI, Spencer CC, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, Strachan DP. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009; 41:1330–1334.
26. Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Pare P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009; 41:216–220.

27. Stremmel W, Gauss A. Lecithin as a therapeutic agent in ulcerative colitis. Dig Dis. 2013; 31:388–390.

28. Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005; 54:966–971.

29. Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology. 2006; 130:S78–S90.

30. Pickard KM, Bremner AR, Gordon JN, MacDonald TT. Microbial-gut interactions in health and disease. Immune responses. Best Pract Res Clin Gastroenterol. 2004; 18:271–285.
31. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005; 307:1915–1920.

32. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005; 308:1635–1638.

33. Eckburg PB, Relman DA. The role of microbes in Crohn's disease. Clin Infect Dis. 2007; 44:256–262.

34. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007; 104:13780–13785.

35. Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016; 14:127–138.

36. Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:661–673.

37. Mitsuyama K, Niwa M, Takedatsu H, Yamasaki H, Kuwaki K, Yoshioka S, Yamauchi R, Fukunaga S, Torimura T. Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol. 2016; 22:1304–1310.
38. Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001; 411:599–603.

39. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006; 314:1461–1463.

40. Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005; 206:260–276.

41. Yang JY, Kim MS, Kim E, Cheon JH, Lee YS, Kim Y, Lee SH, Seo SU, Shin SH, Choi SS, Kim B, Chang SY, Ko HJ, Bae JW, Kweon MN. Enteric viruses ameliorate gut inflammation via Toll-like receptor 3 and Toll-like receptor 7-mediated interferon-beta production. Immunity. 2016; 44:889–900.

42. Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DP, Brown GD, Underhill DM. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012; 336:1314–1317.

43. Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, Berg D, Schukken Y, Scherl E, Simpson KW. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 2007; 1:403–418.

44. Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williamsm HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004; 127:80–93.

45. Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, Alpern JA, Akyildiz A, Theiss AL, Nusrat A, Klapproth JM. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007; 87:1042–1054.

46. Darfeuille-Michaud A. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn's disease. Int J Med Microbiol. 2002; 292:185–193.

47. Martinez-Medina M, Aldeguer X, Lopez-Siles M, Gonzalez-Huix F, Lopez-Oliu C, Dahbi G, Blanco JE, Blanco J, Garcia-Gil LJ, rfeuille-Michaud A. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm Bowel Dis. 2009; 15:872–882.

48. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006; 55:205–211.

49. Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004; 127:412–421.

50. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011; 331:337–341.

51. Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012; 30:759–795.

52. Feuerer M, Hill JA, Kretschmer K, von BH, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci U S A. 2010; 107:5919–5924.

53. Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009; 106:19256–19261.

54. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008; 105:16731–16736.

55. Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009; 15:653–660.

56. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.

57. Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Lowenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–118.

58. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014; 8:1569–1581.

59. Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004; 126:520–528.

60. Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn's disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014; 20:21–35.

61. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34:637–650.

62. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801.

63. Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009; 137:209–220.

64. Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011; 140:1704–1712.

65. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm PL, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D'Amato M, De JD, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De VM, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Hostmicrobe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012; 491:119–124.

66. Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis. 2014; 20:166–175.

67. Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De L, Briggs VJ, Gunther S, Prescott NJ, Onnie CM, Hasler R, Sipos B, Folsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007; 39:207–211.

68. Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007; 39:596–604.

69. Libioulle C, Louis E, Hansoul S, Sandor C, Farnir F, Franchimont D, Vermeire S, Dewit O, de VM, Dixon A, Demarche B, Gut I, Heath S, Foglio M, Liang L, Laukens D, Mni M, Zelenika D, Van GA, Rutgeerts P, Belaiche J, Lathrop M, Georges M. Novel Crohn disease locus identified by genome-wide association maps to a gene desert on 5p13.1 and modulates expression of PTGER4. PLoS Genet. 2007; 3:e58.

70. Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, McArdle W, Strachan D, Bethel G, Bryan C, Lewis CM, Deloukas P, Forbes A, Sanderson J, Jewell DP, Satsangi J, Mansfield JC, Cardon L, Mathew CG. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007; 39:830–832.

71. Moon CM, Shin DJ, Kim SW, Son NH, Park A, Park B, Jung ES, Kim ES, Hong SP, Kim TI, Kim WH, Cheon JH. Associations between genetic variants in the IRGM gene and inflammatory bowel diseases in the Korean population. Inflamm Bowel Dis. 2013; 19:106–114.

72. Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006; 25:309–318.

73. Vong L, Ferraz JG, Panaccione R, Beck PL, Wallace JL. A pro-resolution mediator, prostaglandin D(2), is specifically up-regulated in individuals in long-term remission from ulcerative colitis. Proc Natl Acad Sci U S A. 2010; 107:12023–12027.

74. Friedman DJ, Kunzli BM, Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009; 106:16788–16793.

75. Dieckgraefe BK, Korzenik JR, Anant S. Growth factors as treatment options for intestinal inflammation. Ann N Y Acad Sci. 2006; 1072:300–306.

76. Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013; 8:477–512.

78. Truelove S. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Gastroenterologia. 1954; 81:86–90.

79. Flammer JR, Rogatsky I. Minireview: Glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol Endocrinol. 2011; 25:1075–1086.

80. Kim DH, Cheon JH, Park JJ, Yoon JY, Moon CM, Hong SP, Kim TI, Kim WH. Clinical outcomes and predictive factors for response after the first course of corticosteroid therapy in patients with Crohn's disease. Gut Liver. 2013; 7:58–65.

81. Kornbluth A, Present DH, Lichtiger S, Hanauer S. Cyclosporin for severe ulcerative colitis: a user's guide. Am J Gastroenterol. 1997; 92:1424–1428.
82. Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M, Hanauer SB, McDonald JW. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. N Engl J Med. 2000; 342:1627–1632.

83. Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med. 1995; 332:292–297.
84. Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992; 43:329–339.

85. Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, Hildner K, Bartsch B, Holtmann M, Blumberg R, Walczak H, Iven H, Galle PR, Ahmadian MR, Neurath MF. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003; 111:1133–1145.

86. Wildenberg ME, Koelink PJ, Diederen K, Te Velde AA, Wolfkamp SC, Nuij VJ, Peppelenbosch MP, Nobis M, Sansom OJ, Anderson KI, van der Woude CJ, D'Haens GR, van den Brink GR. The ATG16L1 risk allele associated with Crohn's disease results in a Rac1-dependent defect in dendritic cell migration that is corrected by thiopurines. Mucosal Immunol. 2016; DOI: 10.1038/mi.2016.65.

87. Oancea I, Movva R, Das I, guirre de CD, Schreiber V, Yang Y, Purdon A, Harrington B, Proctor M, Wang R, Sheng Y, Lobb M, Lourie R, Cuiv O, Duley JA, Begun J, Florin TH. Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut. 2017; 66:59–69.

88. Dewit O, Moreels T, Baert F, Peeters H, Reenaers C, de VM, Van HP, Muls V, Veereman G, Mana F, Van OM, Holvoet J, Naegels S, Piessevaux H, Horsmans Y, Gala JL. Limitations of extensive TPMT genotyping in the management of azathioprine-induced myelosuppression in IBD patients. Clin Biochem. 2011; 44:1062–1066.

89. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, Haritunians T, Ye BD, Kim KJ, Park SH, Park SK, Yang DH, Dubinsky M, Lee I, McGovern DP, Liu J, Song K. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020.

90. Kim HS, Cheon JH, Jung ES, Park J, Aum S, Park SJ, Eun S, Lee J, Ruther U, Yeo GS, Ma M, Park KS, Naito T, Kakuta Y, Lee JH, Kim WH, Lee MG. A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD. Gut. 2016; DOI: 10.1136/gutjnl-2016-311921.
91. Melmed GY, Targan SR. Future biologic targets for IBD: potentials and pitfalls. Nat Rev Gastroenterol Hepatol. 2010; 7:110–117.

92. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999; 340:1398–1405.

93. Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999; 10:387–398.

94. Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997; 107:353–358.

95. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003; 10:45–65.

96. Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014; 7:6–19.

97. Holtmann MH, Douni E, Schutz M, Zeller G, Mudter J, Lehr HA, Gerspach J, Scheurich P, Galle PR, Kollias G, Neurath MF. Tumor necrosis factor-receptor 2 is up-regulated on lamina propria T cells in Crohn's disease and promotes experimental colitis in vivo. Eur J Immunol. 2002; 32:3142–3151.

98. Perrier C, de HG, Cremer J, Vermeire S, Rutgeerts P, Van AG, Szymkowski DE, Ceuppens JL. Neutralization of membrane TNF, but not soluble TNF, is crucial for the treatment of experimental colitis. Inflamm Bowel Dis. 2013; 19:246–253.

99. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.

100. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.

101. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333.

102. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.

103. Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, Hanauer SB, Lichtenstein GR, Present D, Sands BE, Sandborn WJ. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.

104. Evans AT, Lee SD. A review and expert opinion of the use of certolizumab for Crohn's disease. Expert Opin Biol Ther. 2012; 12:363–370.

105. Lowenberg M, de Boer NK, Hoentjen F. Golimumab for the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014; 7:53–59.

106. Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van MC, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003; 124:1774–1785.

107. Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, Tremaine WJ, Johnson T, Diehl NN, Zinsmeister AR. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001; 121:1088–1094.

108. Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004; 53:1295–1302.

109. Vos AC, Wildenberg ME, Duijvestein M, Verhaar AP, van den Brink GR, Hommes DW. Anti-tumor necrosis factor-alpha antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology. 2011; 140:221–230.

110. Vos AC, Wildenberg ME, Arijs I, Duijvestein M, Verhaar AP, de HG, Vermeire S, Rutgeerts P, van den Brink GR, Hommes DW. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis. 2012; 18:401–408.

111. Bhol KC, Tracey DE, Lemos BR, Lyng GD, Erlich EC, Keane DM, Quesenberry MS, Holdorf AD, Schlehuber LD, Clark SA, Fox BS. AVX-470: a novel oral anti-TNF antibody with therapeutic potential in inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:2273–2281.
112. Vandenbroucke K, de HH, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van HJ, Demetter P, Steidler L, Remaut E, Cuvelier C, Rottiers P. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010; 3:49–56.

113. Assier E, Semerano L, Duvallet E, Delavallee L, Bernier E, Laborie M, Grouard-Vogel G, Larcier P, Bessis N, Boissier MC. Modulation of anti-tumor necrosis factor alpha (TNF-alpha) antibody secretion in mice immunized with TNF-alpha kinoid. Clin Vaccine Immunol. 2012; 19:699–703.

114. Monteleone I, Pallone F, Monteleone G. Th17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Med. 2011; 9:122.

115. Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009; 15:199–207.

116. Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, Caprioli F, Del Vecchio BG, Paoluzi OA, Macdonald TT, Pallone F, Monteleone G. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology. 2007; 132:166–175.

117. Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res. 2016; 8:2490–2497.
118. Reinisch W, de VW, Bene L, Simon L, Racz I, Katz S, Altorjay I, Feagan B, Riff D, Bernstein CN, Hommes D, Rutgeerts P, Cortot A, Gaspari M, Cheng M, Pearce T, Sands BE. Fontolizumab in moderate to severe Crohn's disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm Bowel Dis. 2010; 16:233–242.

119. Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012; 61:1693–1700.

120. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009; 206:1465–1472.

121. Ueno A, Ghosh A, Hung D, Li J, Jijon H. Th17 plasticity and its changes associated with inflammatory bowel disease. World J Gastroenterol. 2015; 21:12283–12295.

122. Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep. 2008; 10:568–575.

123. Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016; 375:1946–1960.

124. Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, Quezado M, Yang Z, Neurath MF, Salfeld J, Veldman GM, Schwertschlag U, Strober W. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004; 351:2069–2079.

125. Sands BE, Jacobson EW, Sylwestrowicz T, Younes Z, Dryden G, Fedorak R, Greenbloom S. Randomized, double-blind, placebo-controlled trial of the oral interleukin-12/23 inhibitor apilimod mesylate for treatment of active Crohn's disease. Inflamm Bowel Dis. 2010; 16:1209–1218.

126. Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996; 184:241–251.

127. Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003; 63:6042–6050.
128. Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000; 289:1352–1355.

129. Danese S, Grisham M, Hodge J, Telliez JB. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016; 310:G155–G162.

130. Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009; 228:273–287.

131. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012; 367:616–624.

132. Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014; 12:1485–1493.
133. Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008; 1:Suppl 1. S50–S53.
134. Monteleone G, Neurath MF, Ardizzone S, Di SA, Fantini MC, Castiglione F, Scribano ML, Armuzzi A, Caprioli F, Sturniolo GC, Rogai F, Vecchi M, Atreya R, Bossa F, Onali S, Fichera M, Corazza GR, Biancone L, Savarino V, Pica R, Orlando A, Pallone F. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N Engl J Med. 2015; 372:1104–1113.

135. Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R, Bressler B, Geboes K, Schreiber S, Aranda R, Gujrathi S, Luo A, Peng Y, Salter-Cid L, Hanauer SB. Abatacept for Crohn's disease and ulcerative colitis. Gastroenterology. 2012; 143:62–69.

136. Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van DS, Goldblum R, Despain D, Hogge GS, Rutgeerts P. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005; 353:1912–1925.

137. Maas RP, Muller-Hansma AH, Esselink RA, Murk JL, Warnke C, Killestein J, Wattjes MP. Drug-associated progressive multifocal leukoencephalopathy: a clinical, radiological, and cerebrospinal fluid analysis of 326 cases. J Neurol. 2016; 263:2004–2021.

138. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013; 369:711–721.

139. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van AG, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.

140. Vermeire S, O'Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A, Baumgart DC, Schreiber S, Dotan I, Sandborn WJ, Tew GW, Luca D, Tang MT, Diehl L, Eastham-Anderson J, De HG, Perrier C, Egen JG, Kirby JA, van AG, Rutgeerts P. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014; 384:309–318.

141. Sandborn W, Lee SD, Tarabar D, Louis E, Klopocka M, Klaus J, Reinisch W, Hebuterne X, Park DI, Schreiber S. 825 Anti-MAdCAM-1 antibody (PF-00547659) for active refractory Crohn's disease: Results of the OPERA study. Gastroenterology. 2015; 148:S-162.

142. Reinisch W, Sandborn W, Danese S, Cataldi F, Hebuterne X, Salzberg B, Klopocka M, Tarabar D, Vanasek T, Gregus M. 901a A randomized, multicenter double-blind, placebo-controlled study of the safety and efficacy of anti-MAdCAM antibody PF-00547659 (PF) in patients with moderate to severe ulcerative colitis: Results of the TURANDOT study. Gastroenterology. 2015; 148:S-1193.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download