Abstract
We investigated whether diclofenac could influence the development of antigen-presenting cells in an oxygenated cholesterol-rich environment by determining its effects on the 27-hydroxycholesterol (27OHChol)-induced differentiation of monocytic cells into mature dendritic cells (mDCs). Treatment of human THP-1 monocytic cells with diclofenac antagonized the effects of 27OHChol by attenuating dendrite formation and cell attachment and promoting endocytic function. Diclofenac inhibited the transcription and surface expression of the mDC markers of CD80, CD83, and CD88, and reduced the 27OHChol-induced elevation of surface levels of MHC class I and II molecules to the basal levels in a dose-dependent manner. It also reduced the expression of CD197, a molecule involved in DC homing and migration. These results indicate that diclofenac inhibits the differentiation of monocytic cells into mDCs, thereby potentially modulating adaptive immune responses in a milieu rich in cholesterol oxidation products.
Dendritic cells (DCs) are responsible for the initiation of antigen-specific immune responses. They help the immune system respond to foreign antigens while avoiding the generation of autoimmune responses (12). DCs can be generated in vitro from monocytes using exogenous stimuli. Human blood CD14-positive monocytes differentiate into DCs when cultured in combination with GMCSF and IL-4 (23). LPS, TNF-α, and calcium ionophore rapidly induce the differentiation of blood monocytes into DC-like cells (4). Treatment of the human THP-1 monocytic cell line with ionomycin or 27-hydroxycholesterol (27OHChol) results in differentiation into a mature DC (mDC) phenotype (56). DCs play important roles in the initiation of atherosclerosis. Some signals that promote activation of DCs drive the perpetuation of vascular inflammation by priming specific responses (7).
Diclofenac, a nonsteroidal anti-inflammatory drug, is the preferred therapeutic drug for regional musculoskeletal and neuropathic pain (89). Diclofenac exerts anti-inflammatory effects by inhibiting the activity of cyclooxygenase enzymes, thereby preventing the production of prostaglandins (10). The drug also suppresses the secretion of LPS-induced inflammatory cytokines by murine astrocytes (11) and negatively regulates osteogenic differentiation of human adipose tissue-derived stromal cells (12). Thus, diclofenac influences inflammation and cellular differentiation by regulating the expression of gene products. However, the effects of diclofenac on the differentiation of DCs are unknown.
In the current study, we investigated whether diclofenac could influence adaptive immune responses, particularly in an environment rich in cholesterol oxidation products. We report a novel pharmacological action of diclofenac, the inhibition of the 27OHChol-induced differentiation of monocytic cells into mDCs, as indicated by morphological and functional modifications and the downregulation of cell surface molecules.
THP-1 cells, purchased from American Type Culture Collection (Manassas, VA, USA) were maintained in RPMI 1640 containing 10% FBS in the presence of penicillin and streptomycin. 27OHChol and primary antibodies against CD80, CD83, CD88, CD197, and MHC molecules were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Diclofenac was purchased from ENZO Life Sciences (Farmingdale, NY, USA). FITCconjugated dextran (40 kDa) was purchased from Sigma- Aldrich (St. Louis, MO, USA). Alexa Fluor® 488-conjugated secondary antibodies for FACS analysis were purchased from Invitrogen (Eugene, Oregon, USA).
After incubation with diclofenac and 27OHChol, THP-1 cells were re-suspended in culture medium containing 1 mg/ml FITC-conjugated dextran and incubated for 30 min at 37℃ or 4℃ (for background control). Fluorescence was analyzed using a flow cytometer.
Total RNA was reverse-transcribed for 1 h at 42℃ with Moloney murine leukemia virus reverse transcriptase, followed by quantitative real-time PCR. Real-time PCR was performed in triplicate in 96-well plates containing SYBR® Green PCR Master Mix and 10 pM forward and reverse primers for CD molecules and GAPDH. The primer sequences of CD molecules were forward 5'-TGGTGCTGGCTGGTCTTTC-3' and reverse 5'-CTGTGCCACTTCTTTCACTTCC-3' (CD80); forward 5'-TCCTGAGCTGCGCCTACAG-3' and reverse 5'-GCAGGGCAAGTCCACATCTT-3' (CD83); and forward 5'-GTGGTCCGGGAGGAGTACTTT-3' and reverse 5'-GCCGTTTGTCGTGGCTGTA-3' (CD88, C5AR1). Primers for GAPDH were forward 5'-ATGGGGAAGGTGA AGGTCG-3' and reverse 5'-GGGGTCAT TGATGGCAA CAATA-3'.
THP-1 cells were incubated for 2 h at 4℃ with antibodies against CD80, CD83, CD88, CD197, and MHC class I and II molecules. FACS analysis was performed as previously described (13).
We assessed viability of THP-1 cells after treatment with 5, 10, and 25 µg of diclofenac in the presence of 27OHChol. Viability was not altered by treatment with 27OHChol and diclofenac, which indicated that the treatment did not cause cytotoxicity (Supplementary data).
We examined the role of diclofenac on the morphological changes induced by 27OHChol. Cell attachment, which was increased 18.7-fold after stimulation with 27OHChol, was reduced by treatment with diclofenac in a dose-dependent manner (Fig. 1A). In the microscopic images, we observed that the cells stimulated with 27OHChol assumed an mDC-like star shape with dendrites (Fig. 1B, white arrows). The morphological changes, however, were suppressed by co-treatment with diclofenac. These results indicated that diclofenac influenced the monocytic cell morphological changes that were induced by 27OHChol.
We performed endocytosis assays to determine whether diclofenac affected the 27OHChol-induced functional alteration of monocytic cells (Fig. 2). The percentage of cells exhibiting endocytic activity was reduced from 19.0% to 5.7% in the presence of 27OHChol; however, the reduced activity was restored to 11.6%, 16.5%, and 18.8% by treatment with 5, 10, and 25 µg/ml diclofenac, respectively. These data indicated that treatment with diclofenac rescued the modification to endocytic function caused by 27OHChol.
We examined the expression of mDC-specific markers to investigate if diclofenac affected the differentiation of monocytic cells into mDCs. The transcript levels of the mDC markers CD80 , CD83 , and CD88 (C5AR1) were significantly elevated in the presence of 27OHChol, but diclofenac inhibited the transcription of the CD molecules in a dose-dependent manner (Fig. 3A). The transcript levels of CD80 were elevated 11.21-fold in the presence of 27OHChol, but that elevation was reduced to 9.06-, 5.97-, and 1.84-fold by treatment with 5, 10, and 25 µg/ml diclofenac, respectively. CD83 transcripts were elevated 11.48-fold, but reduced to 9.28-, 6.01-, and 2.16-fold by 5, 10, and 25 µg/ml diclofenac, respectively. In addition, CD88 (C5AR1) transcripts, elevated to 13.05-fold by 27OHChol, were reduced to 11.74-, 5.89-, and 3.25-fold by 5, 10, and 25 µg/ml diclofenac, respectively.
We also examined the effects of diclofenac on the surface expression of mDC markers. In agreement with the results of the real time-PCR, flow cytometric analyses showed that diclofenac downregulated cell surface levels of the CD molecules in a dose-dependent manner (Fig. 3B). The percentage of CD80-positive cells was increased from 3.5% to 17.4% in the presence of 27OHChol, but was reduced to 7.9%, 7.2%, and 3.5% by treatment with 5, 10, and 25 µg/ml diclofenac, respectively. CD83-positive cells were increased to 20.0%, but reduced to 9.5%, 8.7%, and 2.7% by 5, 10, and 25 µg/ml diclofenac, respectively. CD88-positive cells were increased to 15.8% by 27OHChol, which was reduced to 4.8%, 3.2%, and 1.7% by treatment with 5, 10, and 25 µg/ml diclofenac, respectively. These results indicated that diclofenac inhibited the transcription and surface expression of mDC markers that were enhanced by 27OHChol.
We performed flow cytometry to examine the effects of diclofenac on the levels of surface MHC molecules (Fig. 4). The 27OHChol-induced increased levels of MHC molecules were downregulated by treatment with diclofenac. The percentage of MHC class I-positive cells was increased from 3.5% to 16.7% by 27OHChol, but was reduced to 15.9%, 12.7%, and 6.4% by treatment with 5, 10, and 25 µg/ml diclofenac, respectively. The percentage of MHC class II-positive cells increased to 20.1% with 27OHChol treatment, and was reduced to 19.4%, 15.4%, and 7.2% in the presence of 5, 10, and 25 µg/ml diclofenac, respectively. These results indicate that diclofenac inhibited the surface expression of MHC molecules that was induced by the oxysterol.
CD197 (CCR7) is highly expressed on mDCs (14). We examined whether diclofenac affected the expression of CD197, a homing molecule, by flow cytometry (Fig. 5). The percentage of CD197-positive monocytic cells was increased from 5.8% to 21.8% after stimulation with 27OHChol, but was decreased to 14.4%, 14.6%, and 8.8% by treatment with 5, 10, and 25 µg/ml diclofenac, respectively. These data indicated that diclofenac attenuated the expression of a molecule involved in DC homing to secondary lymphoid organs.
mDCs are defined by their key morphological features and cell surface phenotype. The key morphological characteristic of DCs is the presence of numerous membrane processes that extend from the main cell body (141516). We, therefore, investigated whether diclofenac affected THP-1 morphology and found that it inhibited the 27OHChol-induced formation of dendrites. The extent of endocytosis, an active transport through which DCs capture antigenic molecules (17), varies depending on the maturation stage of DCs. Antigen uptake activity by mDCs is low (114). We found that endocytic activity was impaired in the presence of 27OHChol, but restored by treatment with diclofenac. These results suggest that diclofenac suppresses DC differentiation from the morphological and functional points of view.
Markers specific for mDCs are involved in the activation of T and B lymphocytes. CD80, known as B7-1 or CD28 ligand, is a surface molecule involved in the activation of B lymphocytes (18). CD83, a co-activator of T lymphocytes, is required for the development of CD4 T cells (19). CD88, known as C5a receptor, plays key roles in the secretion of IL-12 and in Th2 immune responses (20). The suppression of these markers by diclofenac indicates that it can negatively regulate the activation of T and B lymphocytes. Given that activated T and B lymphocytes are involved in atherosclerosis in a milieu rich in cholesterol oxidation products (2122), the results of the current study also suggest that diclofenac could affect the pathogenesis of the disease.
We demonstrated that treatment with diclofenac downregulated the expression of MHC molecules and CD197 on the cell surface. MHC molecules, which are highly expressed on mDCs but not on monocytes or immature DCs, present antigens to stimulate T cells (1). The downregulation of surface MHC molecules suggests that the drug can affect antigen presentation and T cell activation. CD197 is involved in the migration of mDCs into secondary lymphoid organs, such as the spleen. This molecule specifically interacts with CCL19 and CCL21, which are expressed in endothelial cells and the T cell zone of lymphoid organs (232425). After migration, mDCs stimulate naïve T cells in the secondary lymph organs. The downregulation of surface CD197 suggests that diclofenac can impair the migration of mDCs. Therefore, the results of the current study suggest that diclofenac can attenuate T cell activation via the downregulation of MHC molecules and the impairment of mDC migration.
It has been reported that diclofenac has an inhibitory effect on DC2 polarization. Exposure of DC to nickel results in DC maturation and secretion of both type 1 and type 2 cytokines, and diclofenac inhibits nickel-induced production of type 2 cytokine, like CXCL8 and CCL17, without affecting expression of CD83 and CD86 (26). Results of the current study mean that diclofenac inhibits 27OHChol-induced DC maturation, as evidenced by reduced levels of CD80, CD83, CD88 and CD197, and MHC class molecules. Taken together, these findings suggest that diclofenac can be used for regulation of polarization or maturation of DC in response to distinct stimuli.
In this study, we demonstrated that diclofenac inhibits the 27OHChol-induced differentiation of monocytic cells to mDCs. These findings suggest that diclofenac can attenuate T cell-mediated immune responses in a milieu rich in oxidatively modified cholesterol molecules. Further study is necessary in order to assess the in vivo effects of diclofenac on T cell responses in animal models.
Figures and Tables
Figure 1
Effects of diclofenac on cell attachment and morphological changes. (A) After incubation of THP-1 cells (1×106 cells/60-mm culture dish) for 48 h with or without 5, 10, or 25 µg/ml diclofenac in the presence of 27OHChol (2.5 µg/ml), the adherent cells were counted. Data are expressed as mean±SD (n=3 replicates/group). ###p<0.001 vs. control; ***p<0.001 vs. 27OHChol. (B) After incubation with 27OHChol (2.5 µg/ml) for 48 h with or without 5, 10, or 25 µg/ml diclofenac, THP-1 cells were visualized through a microscope. Df, diclofenac.
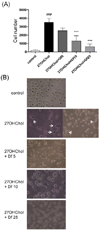
Figure 2
Effects of diclofenac on functional changes in monocytic cells induced by 27OHChol. After incubation for 48 h with 27OHChol (2.5 µg/ml) with or without 5, 10, or 25 µg/ml diclofenac, THP-1 cells were treated with 1 mg/ml FITC-conjugated dextran for 1 h. Cells were analyzed by flow cytometry. Results are the representative of three independent experiments. Df, diclofenac.

Figure 3
Effects of diclofenac on the transcription and surface expression of mDC markers induced by 27OHChol. (A) THP-1 cells (1<106 cells/60-mm culture dish) were cultured for 48 h with or without 5, 10, or 25 µg/ml diclofenac in the presence of 27OHChol. Transcript levels of CD80, CD83, and CD88 genes were analyzed by real-time PCR. Data are expressed as mean±SD (n=3 replicates/group). ###p<0.001 vs. control; ***p<0.001 vs. 27OHChol. (B) THP-1 cells were cultured for 48 h in the presence of 2.5 µg/ml 27OHChol with or without the indicated concentrations of diclofenac. Cells were analyzed by flow cytometry after immunostaining with antibodies against CD80, CD83, and CD88. Results are the representative of three independent experiments. Df, diclofenac.
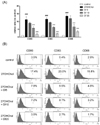
Figure 4
Effects of diclofenac on the expression of MHC class I and II molecules induced by 27OHChol. After incubation for 48 h with 27OHChol (2.5 µg/ml) with or without 5, 10, or 25 µg/ml diclofenac, the stimulated THP-1 cells (1×106 cells/60 mm-culture dish) were immunostained for MHC class I and II. Fluorescence was analyzed by flow cytometry. Results are the representative of three independent experiments. Df, diclofenac.

Figure 5
Diclofenac reduced the expression of the homing molecule CD197 induced by 27OHChol on monocytic cells. THP-1 cells (1×106 cells/60-mm culture dish) were cultured for 48 h with 2.5 mg/ml 27OHChol with or without the indicated concentrations of diclofenac. The harvested cells were immunostained with antibodies against CD197 and analyzed by flow cytometry. Results are the representative of three independent experiments. Df, diclofenac.

References
1. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991; 9:271–296.

2. Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U. S. A. 1996; 93:2588–2592.

3. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994; 179:1109–1118.

4. Lyakh LA, Koski GK, Telford W, Gress RE, Cohen PA, Rice NR. Bacterial lipopolysaccharide, TNF-alpha, and calcium ionophore under serum-free conditions promote rapid dendritic cell-like differentiation in CD14+ monocytes through distinct pathways that activate NK-kappa B. J Immunol. 2000; 165:3647–3655.

5. Berges C, Naujokat C, Tinapp S, Wieczorek H, Hoh A, Sadeghi M, Opelz G, Daniel V. A cell line model for the differentiation of human dendritic cells. Biochem Biophys Res Commun. 2005; 333:896–907.

6. Son Y, Kim SM, Lee SA, Eo SK, Kim K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochem Biophys Res Commun. 2013; 438:161–168.

8. Kvien TK, Viktil K. Pharmacotherapy for regional musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003; 17:137–150.

10. Harder AT, An YH. The mechanisms of the inhibitory effects of nonsteroidal anti-inflammatory drugs on bone healing: a concise review. J Clin Pharmacol. 2003; 43:807–815.

11. Al-Amin MM, Uddin MM, Rahman MM, Reza HM, Rana MS. Effect of diclofenac and antidepressants on the inflammatory response in astrocyte cell culture. Inflammopharmacology. 2013; 21:421–425.

12. Hadjicharalambous C, Alexaki VI, Alpantaki K, Chatzinikolaidou M. Effects of NSAIDs on the osteogenic differentiation of human adipose tissue-derived stromal cells. J Pharm Pharmacol. 2016; 68:1403–1408.

13. Son Y, Kim BY, Eo SK, Park YC, Kim K. Dexamethasone suppresses oxysterol-induced differentiation of monocytic cells. Oxid Med Cell Longev. 2016; 2016:2915382.

14. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000; 18:767–811.

15. Lord RS, Bobryshev YV. Clustering of dendritic cells in athero-prone areas of the aorta. Atherosclerosis. 1999; 146:197–198.
16. Ozmen J, Bobryshev YV, Lord RS, Ashwell KW. Identification of dendritic cells in aortic atherosclerotic lesions in rats with diet-induced hypercholesterolaemia. Histol Histopathol. 2002; 17:223–237.
18. Katz FE, Parkar M, Stanley K, Murray LJ, Clark EA, Greaves MF. Chromosome mapping of cell membrane antigens expressed on activated B cells. Eur J Immunol. 1985; 15:103–106.

19. Fujimoto Y, Tu L, Miller AS, Bock C, Fujimoto M, Doyle C, Steeber DA, Tedder TF. CD83 expression influences CD4+ T cell development in the thymus. Cell. 2002; 108:755–767.

20. Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995; 182:1527–1536.

21. Perry HM, McNamara CA. Refining the role of B cells in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012; 32:1548–1549.

22. Profumo E, Buttari B, Saso L, Capoano R, Salvati B, Rigano R. T lymphocyte autoreactivity in inflammatory mechanisms regulating atherosclerosis. ScientificWorldJournal. 2012; 2012:157534.

23. Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008; 29:325–342.

24. Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U. S. A. 2000; 97:12694–12699.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download