Abstract
Most allergic diseases are caused by activation of Th2 type immune responses resulting in the production of specific IgE against proteins found in normally harmless substances such as pollen, mites, epithelia or food. Allergenic substances are composed, in addition to proteins, of other compounds such as carbohydrates and lipids. Those lipids are able to promote the development of Th2-type responses associated with allergy. There are lipids found in pollen, milk or insect venom that are specifically recognized by CD1 restricted unconventional T lymphocytes, which can promote allergic reactions. Furthermore, a large number of allergens are proteins containing hydrophobic parts that specifically bind lipids that are capable to favor allergenic immune responses. Also, lipids associated to substances like pollen, dander, epithelia or the bacteria can act on cells of the innate system, including dendritic cells, which in turn lead to the differentiation of Th2-type clones. Finally, lipids may also influence the ability of allergens to be exposed to the immune system within the oral, respiratory or intestinal mucosa where allergic response occurs with great frequency.
Allergies are a group of complex diseases characterized by an abnormal immune response or hypersensitivity to foreign but harmless substances or antigens called allergens. Most of the innocuous materials to which we are exposed to every day, such as food, pollen, dander, animal epithelia, mites etc. are unable to produce allergy. Only a very small fraction of them are able to trigger allergic responses in predispose individuals. In most cases the immune mechanism underlying the allergic reaction encompasses an adaptive Th2 type response, subsequently leading to the production of allergen specific IgE. The vast majority of allergens that are recognized specifically by both Th2 cells and IgE molecules are proteins, but we do not know why a particular protein becomes an allergen. Although most of the major allergens have limited structural or functional characteristics, for example, there seems to be an overrepresentation of proteins which are proteolytic enzymes, it is clear that none of them could be associated with the allergenicity of a protein (1).
The simplistic view of the immune system distinguishing self from non-self has been completed in recent years by adding the concept of danger or stress. A substance must be accompanied by a signal of stress or danger that is normally recognized by the innate immune system before an adaptive response can be launched (2). In the case of viruses or bacteria, the most usual reaction involves the activation of Th1 or Th17 cells and the subsequent production of IgG or IgA. In contrast, most allergens as well as helminths, activate Th2 cells resulting in the production of IgE (3). The mechanisms of the innate immune response that promote a Th2 type activation are not completely understood, but substances acting as adjuvants can condition them. Allergy inducing substances are accompanied by non-proteinaceous elements such as carbohydrates or lipids that can promote skewing of the immune response to a Th2 profile, interestingly, among major allergens there is a high frequency of lipid-binding proteins. In this review, we will analyze the role of lipids in the development of allergies, a concept that has only recently begun to be considered.
There are few examples describing immune adaptive recognition of lipids by the immune system, e.g. specific IgE or positive responses in skin prick test. It has been described that allergic individuals have specific IgE and positive prick cutaneous tests against phospholipids isolated from cypress pollen (4). There are also reports suggesting that small lipids may behave as haptens capable of activating T helper lymphocytes. α-acaridial, a monoterpene small lipid from house dust mites was identified as one of the components which are able to induce delayed hypersensitivity reactions in mice (5). Lipids found in allergenic substances are not the main target of the adaptive immune system, but they can influence the development of specific allergic reaction through different mechanisms.
In the allergic immune response, the antigenic proteins are processed and presented as small peptides by the highly polymorphic MHC molecules to T-helper lymphocytes that in turn interact with B cells and facilitate their differentiation to IgE producing plasma cells. CD1 molecules are structurally very similar to MHC class I molecules, but they are largely not polymorphic (6) and instead of peptides, they present lipids or glycolipids to specialized T lymphocytes (7). These unconventional T-cells may act as adjuvants producing an environment of Th2-realted cytokines that help to induce IgE class-switch by B-cells.
CD1 proteins are lipid-presenting molecules expressed on dendritic cells (DCs), macrophages, B cells and some epithelial cells. CD1 molecules can be divided in two groups: group I encloses CD1a, b and c, and group II contains CD1d that is recognized by a subset of T lymphocytes named Natural Killer T cells (NKT cells) (8). There are two types of NKT cells: type I NKT cells or invariant NKT cells (iNKT cells) which use an invariant TCRα-chain Vα24 with the Jα18 joining region, which associate with Vβ11-chain (9). In the other hand Type II NKT cells recognize also lipids presented by CD1d but they use a wide diverse array of TCRs. iNKT cells are cytotoxic, and they are able to produce Th1 and Th2 cytokines in response to glycolipids from bacteria and from mammals, including self-lipids. The most potent activator of iNKT cells is α-Galactosilceramide (α-GalCer), a compound initially isolated from a marine sponge (10). Now we know that CD1d present to iNKT cells a variety of microbe-derived glycolipidic ligands, including glycosphingolipids (GSL) from Sphingomonas (11), glycodiacly-glycerols from Borrelia burgdorferi and Ehrlichia muris (12), or lipophosphoglycan from Leishmania (13). Mammalian glycolipids such as GPI (14), iGb3 (15), phospholipids (PI, PC, PG and PE) (16) tumor-derived glycolipids (disialoganglioside GD3) (17) and β-d-glucopyranosyl-ceramide (β-GlcCer) (18) have also been described to be presented to NKT cells. Because of its capacity to produce both Th1 and Th2 cytokines and to influence innate and adaptive immune responses, iNKTs are considered as regulatory T cells which can be involved in responses against infections (19), cancer as well as in autoimmune and allergic diseases (20). In humans, conflicting results regarding the relationship between NKT cells and allergic asthma have been published. Some reports showed high levels of iNKT cells in bronchial samples from asthmatic patients (2122232425). On the other hand, other studies did not observe significant differences in the numbers of iNKT cells when comparedbetween controls and patients with asthma (262728). A possible explanation for these discrepancies may lie in the fact that NKT cells may act only transiently to induce a long-term Th2 allergic response against proteins, as observed in a OVA-specific mouse model of asthma (29). In addition, a number of studies performed in animal model of allergic asthma models strongly suggest a crucial function of NKT cells (29303132) which may act as adjuvant to enhance Th2 inflammatory responses in the airways (33). Lipids isolated from common allergenic substances have been demonstrated to activate NKT-cells (Table I). We have shown that polar lipids, free fatty acids, diacylglycerols, as well as triacylglycerols isolated from pollen grains are able to specifically act on human dendritic cells (Fig. 1) activating the peroxisome proliferator activated-receptor γ (PPARγ) which results in up-regulation of CD1d molecule on their cell surface and the subsequent activation of NKT cells (58). Furthermore, the activation and up-regulation of CD1d by pollen-derived lipids also occur in other types of antigen presenting cells such as macrophages and B-cells. Interestingly, monocytes treated with pollen lipids were able to stimulate iNKT cells with no need of CD1d upregulation, suggesting that lipids from olive pollen can be presented directly by CD1d molecules to iNKT cells (34). Not only pollen lipids are recognized by NKT cells, lipids derived from pathogens have been described to mobilize NKT capable to induce allergic responses. The GSL-1 lipid glycosphingolipid GSL-1, present in Gram-negative bacteria that lack LPS, such as genus Sphingomonas, is an agonist ligand of mouse and human NKT cells which was able to sensitize mice to OVA in a model of allergic airway hyperreactivity, in a CD1d and NKT-dependent way (31). Aspergillus fumigatus is an ubiquitous and opportunistic fungus as well as an important allergen in human and animals. Asperamide B, a glycosphingolipid from A. fumigatus is able to induce airway hyperreactivity by activating pulmonary iNKT cells in an IL-33 dependent way in mice. Asperamide B binds to CD1d and activates iNKT cells from mice and humans (35). Food allergens also contain lipids capable to activate NKT cells. In experiments with mice, the seed storage 2S albumin as well as Ber e 1, the main allergen in Brazil nuts, were capable to induce anaphylactic antibodies, IgE and IgG1, only when it was administered together with neutral or common phospholipids extracted from Brazil nuts. Furthermore iNKT deficient mice produced lower levels of Ber e 1 IgE and IgG1 specific antibodies, whereas human CD1d-restricted T-cell lines derived from nut-allergic patients were able to produce IL-4 in response to Ber/lipid C (36). Milk allergy can also be linked to activation of NKT cells. Milk sphingomyelin, but not egg-ceramide, are recognized by iNKT cells, inducing their proliferation and production of Th2 cytokines, especially in children with milk allergy. On the other hand, it has been observed that children with milk allergies have lower numbers of iNKTs in their peripheral blood and that they have a higher Th2 response to αGalcer and milk sphingomyelin than healthy individuals (37). Similar observations were made in children with eosinophilic esophagitis (38).
Beside the role of NKT cells, other types of CD1 restricted T-cells capable to recognize lipids haven been linked to allergic responses in human patients. Activations of CD1a and CD1d-restricted TCRαβ+ and TCRγδ+ T-cells that specifically recognize phospholipids (PLs) from cypress pollen have been described in allergic patients (4). An interesting relationship between allergenic proteins, lipids and CD1a-restricted T-cells have been recently discovered in the field of allergy to venoms. Bourgeois et al. (39), found that stimulatory factors of CD1a restricted T-cells partition into protein-containing fractions of bee venom extracts. Bee venom-derived phospholipase A2 (PLA2) digests common phosphodiacylglycerides to generate small lipidic neoantigens, such as free fatty acids and lysophospholipids. CD1a molecules subsequently present these neoantigens to T-cells that may be involved in generation of the allergic response. Reinforcing this theory, Subramaniam et al. (40), described elevated and cross-responsive CD1a-reactive T cellsamong bee and wasp venom allergic individuals suggesting common components of allergenicity in both species.
In summary, unconventional T cells recognizing lipids or glycolipids presented by CD1 molecules play important roles in allergic reaction. They use receptors formed by somatic recombination, as do their partners, the conventional T cells, however they can be considered as innate or innate-like cells because they recognize a limited number of antigens, they use a much more restricted TCR repertoire and probably do not develop memory features like conventional T-cells. However, their T-cell characteristics, including their plasticity and ability to regulate and deviate Th1, Th2 and Th17 responses, make unconventional T-cells very interesting therapeutic targets in order to reverse or treat allergic diseases.
Besides being recognized directly by the adaptive players of the immune response (B-cells or T-cells), lipids may influence the development of allergic process through interaction with the components of innate immune system. These kind of interactions could explain why some proteins are more allergenic than others influencing the way they engage with the host or contributing to the development of a determined cytokine pattern, such as IL-4, IL-5 and IL-13, essentials for triggering Th2 reactions (41).
Lipids may be directly bound to protein allergens or being associated with them in allergenic substances such as pollen. In addition, most of the plant pollen contain bacteria (which bear lipids), and other Pollen Associated Lipid Mediators (PALMS) that can activate innate immune cells, influence TLR signaling, APC maturation and cytokine profile production.
When exposed to water, pollen grains are able to release a number of lipids named pollen-associated lipid mediators (PALMs) which are homologous to eicosanoids, and that can act as adjuvants enhancing the inflammatory responses (Table II) (Fig. 1). A group of PALMS show homology with leukotriene B4 and are able to induce chemotaxis and activation of human neutrophils and eosinophils, in a way that is independent of the sensitization status of the donors (424344). Another group of PALMS are phytoprostanes, such as Pollen-derived Phytoprostanes E1 (PPE1) which show homology to prostaglandin E2 (45) and that are able to inhibit the capacity of DCs to induce Th1 responses, dampening production of IL-12 and Th1-type cytokines (46) while increasing the ability of DCs to cause Th2 cell differentiation and attraction (4748). Phytoprostanes are also capable to interfere with the LPS induced Th1 response of mouse DCs, by inhibiting the IL-6, IL-12 and TNF-α secretion (49).
TLRs are critical players in the innate immune responses that recognized conserved structures such as PAMPs (pathogen-associated molecular patterns) present in microbes, which can be important in allergy development (5051). TLR signaling can enhance both Th1 and Th2 responses. TLR ligands are proteins as well as no proteinaceous ligands like DNA or lipidic molecules. Lipopolysaccharide (LPS) or endotoxin, is found in the membrane of Gram-negative bacteria that binds to TLR4. Exposure to high doses of LPS generally induces Th1 responses, protecting against allergy development, while inhalation of low doses together with the respective allergen favor a Th2 environment and lung inflammation through TLR4 (4352).
The adjuvant activity provided by allergen-associated lipids is common to different allergenic proteins through TLR activation by different mechanisms. Secretoglobins, (small secretory proteins) like cat Fel d 1 or dog lipocalin Can f 6, bind LPS and are able to amplify LPS/TLR signaling (53). The structure of Der p2 a major dust mite allergen, is similar to MD-2, the LPS-binding co-receptor that promotes signaling of TLR4, and it is involved Th2 driven inflammatory responses as revealed in experimental allergic asthma by challenge with Der p2 (51). TLR2 and TLR4 can be activated by lipids (palmitic, oleic, or stearic acids) associated to the cockroach allergen Bla g 1, directing the immune response to allergy (54).
Extracts from ryegrass pollen has highly variable LPS contents, as well as TLR-2 and TLR-9 ligands, which are capable to increase Th1 and Th2 effector cell while decrease regulatory T-cells induction in PBMC (Peripheral Blood Mononuclear Cells) responses of allergic and nonatopic subject (55).
Supernatants containing microbial lipids of gram-positive Bacillus cereus and Bacillus subtilis present on timothy pollen grains augmented the maturation of monocyte-derived immature DCsand inflammatory Th1, Th2 and Th17 responses in donors with grass pollen allergy suggesting that may serve as adjuvants by augmenting DC maturation (56). Aqueous birch pollen extracts, containing PALMS, are able to increase the expression of the CXCR4 chemokine receptor and the reduction of CCR1 and CCR5 expression on immature DCs. They also reduce the levels of Th1 chemokines such as CXCL10, CCL5 and CCL22 chemokines in LPS matured DCs (48). Lipids from olive pollen activate human dendritic cells inducing PPAR-γ activation which results in CD1d upregulation on the cell surface allowing recognition of NKT-cells (57). Furthermore, inside the cell, the lipids enclose the antigen in lipid vesicles that target early endosomes, enhancing the antigen processing and presentation and the subsequent response of T lymphocytes (58).
Proteolytic activity has been proposed to be a functional property of many allergenic proteins, however, specific lipid binding capacity is far more commonly found among major allergens. Allergen-lipid complex occur in many animal and plant food allergens and these lipids include mostly triacylglycerols, phospholipids and cholesteryl ester. Several protein families share these characteristic, including allergens, such as Bet v 1-like proteins (the major allergen of birch pollen), nonspecific lipid transfer proteins (nsLTPs), a family of pan-allergens expressed throughout the plant kingdom, secretoglobins, lipocalins, oleosins, 2S albumins, and mite group 2, 5, and 7 proteins (59606162). The lipids that accompany those proteins could modify Th2 responses, either by promoting the development of an IgE dominated response, for example: Fel d 1 or Pru p 3, or inhibiting them, as it is the case of Bet v 1 (63), however the mechanisms underlying these observations are largely unknown.
There are several examples suggesting a potentially differential immune function of allergenic proteins depending on their loading status. Lipocalins are the most important mammal-derived respiratory allergens, found in dander, salive and urine from cat, dog, horse, and mouse. Some of them display a high sequence similarity between different species, and constitute a group of potentially cross-reactive molecules which may contribute to allergic cross-reactions between species, including humans (64). They usually carry lipids or other hydrophobic compounds such as steroids, hormones vitamins, cofactors, and odorants that may be key for the biological functions of the lipocalin and other allergens (65). It has been shown that the lipocalin Bos d 5, the major cow's milk allergen, in its loaded form harbors immune-suppressive functions, and it is able to induce regulatory T cells. In contrast, in the empty status it is able to direct the response toward Th2 allergic response, interfering with regulatory proteins like human lipocalin homologue (LCN2) (66). Similarly, the pollen allergen Bet v 1 has been found to be structurally related to human lipocalin-2, and it is able to skew the immune system depending also on its loading status. The natural ligand of the main Bet v1 isoform is the glycosylated flavonoid quercetin-3-o-sophoroside, this glycolipid molecule might be released in contact with lipids membranes, where it could participate in transduction process and change the conformation of Bet v 1, mounting an allergic Th2 response (67). However, when applied in a complex with iron-siderophore (quercetin can bind iron molecules), Bet v 1 Th2-skewing potential is inhibited (63). The non-specific lipid transfer proteins (nsLTPs) family, includes aeroallergens, such as Par j 8 from Parietaria Judaica and olive Ole e 7, several important food allergens, like peach (Pru p 3), tree nuts like chestnut (Cas s 8), vegetables and cereals or the skin sensitizer Hev b 12 from latex and pollen (68). The binding of nsLTPS to different types of lipids, including fatty acids, phospholipids, glycolipids and prostaglandin B, could contribute to the activation of innate immune cells, and enhance an IgE dominant response (69).
There are allergens, like the 2S albumin from Brazil nut, Ber e 1 or Oleosins, (from hazelnut, olive fruit, peanut, sesame and soybean,) which have a hydrophobic cavity with potential capacity to bind lipids which is similar in size to that observed in nsLTPs (707172). Oil bodies of oil seeds have also protein-lipid complexes which can influence allergenicity. This is the case of Sin a 2 and Ara h 1, mustard and peanut allergens which bind lipids that may be important to induce allergic responses against their protein components (73).
In the other hand, some treatments of allergens can affect their lipid content. Due to their high polyunsaturated fatty acid content peanuts suffer lipid oxidation,during storage or roasting. Allergenicity could change if new protein-lipid complexes or changes in protein structures occur when the lipid hidroperoxides formed attack the proteins (74).
Lipids can interfere the relationship between the epithelium barrier and the potential allergenic proteins in a wide variety of forms (Fig. 1). The allergen contact with immunocompetent cells under the epithelium may be facilitated by the lipids transported by nSLTPs, enhancing their transfer between membranes (7576). For example, the formation of lipid rafts and caveolas that allow endocytosis in epithelial cells of Prup p3, an nSLTP from peach. In addition, the reduction of Th2 cytokines of hypoallergenic peach nSLTP, compared to Prup p3 is associated with a lower transport capacity of the former (77). Der p2, may be internalized by the human airway epithelium and potentiate the airway inflammation because of its similar structure and function with the myeloid 2 differentiation protein (MD2), a TLR receptor signaling protein (78). Other proteins also bind lipids and can be associated with lipid membranes such as Bet v1-like family (79).
Peanuts contain significant amount of triglycerides, and Li et al. (80), showed that allergic sensitization and anaphylaxis to oral co-administered peanut proteins in mice could be initiated by medium-chain but not long chain triglycerides (LCT). The lipids may affect absorption into blood by stimulating absorption into Peyer patches and secretion of jejunal-epithelial thymic stromal lymphopoietin, interleukin IL25 and IL33 and promoting a Th2 cytokine response.
Digestion provides additional lipids that can protect allergens, and new protein lipid complexes can be formed in the gut when lipids such as bile acids, cholesterol and phosphatidylcholine are mixed mainly by peristalsis for emulsification (81). Phosphatidylcholine (PC) or lecithin, the main component of cell membranes, is a phospholipid produced in the liver and secreted in the bile. It has been shown that secondary structure of cherry, apple or hazelnut allergens homologous of Bet v1 can be changed when PC emulsifies the fat of the diet. Increased production of histamine or local skin inflammation also increases due to the protective effect of PC, decreasing the action of the duodenal enzymes on grape nSLTPs (8283). PC also protects milk allergens like a-lactalbumin and b-lactoglobulin (Bos d5), the major mammalian milk allergen (belonging to the lipocalin protein family), and the induction of IgE and IgG is only possible by native, but not digested β-lactoglobulin (84).
Lipids can also inhibit the capacity to induce allergic responses against proteins. An example of this is the ability of the bacterial lipopolysaccharide (LPS) to protect against allergy induction against the house dust mite (HDM). It has recently been reported (85) that chronic exposure of LPS protects mice of asthma induced by HDM. The authors showed that LPS induces the expression of A20, a regulator of NF-κB activity, in the respiratory epithelial cells. This results in a decrease of DC activating cytokines secreted by epithelial cells which translates in a suppression of the Th2 response to HDM.
In summary, a large fraction of allergenic proteins recognized by IgE bind lipids of different nature that can influence the immune response potentiating Th2 type reactions by mechanisms not yet clarified. In addition, allergens come very often together with lipids contained in pollen, epithelia, dander, food or even bacteria associated to them. These lipids can act on cells of the innate system, including dendritic cells, which in turn lead to the differentiation of Th2-type clones recognizing peptides from the allergenic proteins. Those lipids are also able to influence the ability of allergens to come into contact with the immune system of the oral, respiratory or intestinal mucosa where allergic type response occurs with great frequency. Increasing the knowledge of the different substances and mechanisms that influence the allergenicity of a protein will result in development of better approaches to treat or prevent allergic diseases.
Figures and Tables
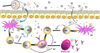 | Figure 1A model of how lipids can influence the allergic response. Particles (1) (pollen, animal hair, food, etc.) containing peptidic (red squares) allergens and lipids (yellow triangles) can be internalized by DCs (2). These cells present lipids (3) to NKT cells or other CD1 restricted T lymphocytes, which release cytokines (4) such as IL4, which in turn promote the differentiation of Th0 lymphocytes into Th2 cells (4) which recognize peptidic antigens through HLA class II molecules. Subsequently, Th2 cells promote the activation of B-cells (5) to develop into IgE producing plasma cells (6). On the other hand, lipids in different forms, for example attached to lipid-carrying proteins (7), PALMS (pollen associated lipids mediators), etc. can influence the relationship between peptidic allergens and a variety of cell types, including respiratory or intestinal epithelial cells (8) or antigen-presenting cells (9), which, by mechanism still undefined (10) , may facilitate the development of Th2-type responses. |
Table I
Lipids interacting with unconventional T-cells

Table II
Lipids interacting with the innate immune system

ACKNOWLEDGEMENTS
Our work was supported by grants from Spanish Ministerio de Sanidad (Ref. FIS-PI08-0125), MINECO (PI13-00218, SAF2016-78711-R) and Universidad Complutense de Madrid (Ref. 920631).
References
1. Dall'antonia F, Pavkov-Keller T, Zangger K, Keller W. Structure of allergens and structure based epitope predictions. Methods. 2014; 66:3–21.
3. Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014; 14:247–259.

4. Agea E, Russano A, Bistoni O, Mannucci R, Nicoletti I, Corazzi L, Postle AD, De LG, Porcelli SA, Spinozzi F. Human CD1-restricted T cell recognition of lipids from pollens. J Exp Med. 2005; 202:295–308.

5. Sasai T, Hirano Y, Maeda S, Matsunaga I, Otsuka A, Morita D, Nishida R, Nakayama H, Kuwahara Y, Sugita M, Mori N. Induction of allergic contact dermatitis by astigmatid mite-derived monoterpene, alpha-acaridial. Biochem Biophys Res Commun. 2008; 375:336–340.

6. Oteo M, Parra JF, Mirones I, Gimenez LI, Setien F, Martinez-Naves E. Single strand conformational polymorphism analysis of human CD1 genes in different ethnic groups. Tissue Antigens. 1999; 53:545–550.

7. Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015; 15:643–654.

9. Birkholz AM, Kronenberg M. Antigen specificity of invariant natural killer T-cells. Biomed J. 2015; 38:470–483.

10. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997; 278:1626–1629.

11. Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005; 434:520–525.

12. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005; 434:525–529.

13. Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, Porcelli SA, Spath GF. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004; 200:895–904.

14. Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998; 279:1541–1544.

15. Zhou D, Mattner J, Cantu C III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004; 306:1786–1789.

16. Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000; 12:211–221.

17. Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003; 198:173–181.

18. Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011; 12:1202–1211.

19. Van Kaer L, Parekh VV, Wu L. The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front Immunol. 2015; 6:226.

20. Subleski JJ, Jiang Q, Weiss JM, Wiltrout RH. The split personality of NKT cells in malignancy, autoimmune and allergic disorders. Immunotherapy. 2011; 3:1167–1184.

21. Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:1117–1129.

22. Pham-Thi N, de BJ, Leite-de-Moraes MC. Invariant natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:2613–2616.

23. Reynolds C, Barkans J, Clark P, Kariyawasam H, Altmann D, Kay B, Boyton R. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J Allergy Clin Immunol. 2009; 124:860–862.

24. Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010; 153:239–248.

25. Hamzaoui A, Cheik RS, Grairi H, Abid H, Ammar J, Chelbi H, Hamzaoui K. NKT cells in the induced sputum of severe asthmatics. Mediators Inflamm. 2006; 2006:71214.

26. Thomas SY, Lilly CM, Luster AD. Invariant natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:2613–2616.

27. Mutalithas K, Croudace J, Guillen C, Siddiqui S, Thickett D, Wardlaw A, Lammas D, Brightling C. Bronchoalveolar lavage invariant natural killer T cells are not increased in asthma. J Allergy Clin Immunol. 2007; 119:1274–1276.

28. Bratke K, Julius P, Virchow JC. Invariant natural killer T cells in obstructive pulmonary diseases. N Engl J Med. 2007; 357:194–195.

29. Scanlon ST, Thomas SY, Ferreira CM, Bai L, Krausz T, Savage PB, Bendelac A. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J Exp Med. 2011; 208:2113–2124.

30. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003; 9:582–588.

31. Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006; 103:2782–2787.

32. Lisbonne M, Diem S, de Castro KA, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, Van EP, Dy M, Askenase P, Russo M, Vargaftig BB, Herbelin A, Leite-de-Moraes MC. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003; 171:1637–1641.

33. Nie H, Yang Q, Zhang G, Wang A, He Q, Liu M, Li P, Yang J, Huang Y, Ding X, Yu H, Hu S. Invariant NKT cells act as an adjuvant to enhance Th2 inflammatory response in an OVA-induced mouse model of asthma. PLoS One. 2015; 10:e0119901.

34. Abos-Gracia B, Lopez-Relano J, Revilla A, Castro L, Villalba M, Martin AB, Regueiro JR, Fernandez-Malave E, Martinez-Naves E, Gomez del. Human invariant natural killer T cells respond to antigen-presenting cells exposed to lipids from Olea europaea pollen. Int Arch Allergy Immunol. 2017; 173:12–22.

35. Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, DeKruyff RH, Savage PB, Umetsu DT. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013; 19:1297–1304.

36. Mirotti L, Florsheim E, Rundqvist L, Larsson G, Spinozzi F, Leite-de-Moraes M, Russo M, Alcocer M. Lipids are required for the development of Brazil nut allergy: the role of mouse and human iNKT cells. Allergy. 2013; 68:74–83.

37. Jyonouchi S, Abraham V, Orange JS, Spergel JM, Gober L, Dudek E, Saltzman R, Nichols KE, Cianferoni A. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011; 128:102–109.

38. Jyonouchi S, Smith CL, Saretta F, Abraham V, Ruymann KR, Modayur-Chandramouleeswaran P, Wang ML, Spergel JM, Cianferoni A. Invariant natural killer T cells in children with eosinophilic esophagitis. Clin Exp Allergy. 2014; 44:58–68.

39. Bourgeois EA, Subramaniam S, Cheng TY, De JA, Layre E, Ly D, Salimi M, Legaspi A, Modlin RL, Salio M, Cerundolo V, Moody DB, Ogg G. Bee venom processes human skin lipids for presentation by CD1a. J Exp Med. 2015; 212:149–163.

40. Subramaniam S, Aslam A, Misbah SA, Salio M, Cerundolo V, Moody DB, Ogg G. Elevated and crossresponsive CD1a-reactive T cells in bee and wasp venom allergic individuals. Eur J Immunol. 2016; 46:242–252.

42. Behrendt H, Kasche A, Ebner von EC, Risse U, Huss-Marp J, Ring J. Secretion of proinflammatory eicosanoid-like substances precedes allergen release from pollen grains in the initiation of allergic sensitization. Int Arch Allergy Immunol. 2001; 124:121–125.

43. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharideenhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.

44. Plotz SG, Traidl-Hoffmann C, Feussner I, Kasche A, Feser A, Ring J, Jakob T, Behrendt H. Chemotaxis and activation of human peripheral blood eosinophils induced by pollen-associated lipid mediators. J Allergy Clin Immunol. 2004; 113:1152–1160.

45. Karg K, Dirsch VM, Vollmar AM, Cracowski JL, Laporte F, Mueller MJ. Biologically active oxidized lipids (phytoprostanes) in the plant diet and parenteral lipid nutrition. Free Radic Res. 2007; 41:25–37.

46. Traidl-Hoffmann C, Kasche A, Jakob T, Huger M, Plotz S, Feussner I, Ring J, Behrendt H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J Allergy Clin Immunol. 2002; 109:831–838.

47. Gutermuth J, Bewersdorff M, Traidl-Hoffmann C, Ring J, Mueller MJ, Behrendt H, Jakob T. Immunomodulatory effects of aqueous birch pollen extracts and phytoprostanes on primary immune responses in vivo. J Allergy Clin Immunol. 2007; 120:293–299.

48. Mariani V, Gilles S, Jakob T, Thiel M, Mueller MJ, Ring J, Behrendt H, Traidl-Hoffmann C. Immunomodulatory mediators from pollen enhance the migratory capacity of dendritic cells and license them for Th2 attraction. J Immunol. 2007; 178:7623–7631.

49. Kamijo S, Takai T, Kuhara T, Tokura T, Ushio H, Ota M, Harada N, Ogawa H, Okumura K. Cupressaceae pollen grains modulate dendritic cell response and exhibit IgEinducing adjuvant activity in vivo. J Immunol. 2009; 183:6087–6094.

50. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–995.

51. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.

52. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007; 178:5375–5382.

53. Herre J, Gronlund H, Brooks H, Hopkins L, Waggoner L, Murton B, Gangloff M, Opaleye O, Chilvers ER, Fitzgerald K, Gay N, Monie T, Bryant C. Allergens as immunomodulatory proteins: the cat dander protein Fel d 1 enhances TLR activation by lipid ligands. J Immunol. 2013; 191:1529–1535.

54. Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, Chapman MD, Tomer KB, London RE, Pomes A. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol. 2013; 132:1420–1426.

55. Mittag D, Varese N, Scholzen A, Mansell A, Barker G, Rice G, Rolland JM, O'Hehir RE. TLR ligands of ryegrass pollen microbial contaminants enhance Th1 and Th2 responses and decrease induction of Foxp3(hi) regulatory T cells. Eur J Immunol. 2013; 43:723–733.

56. Heydenreich B, Bellinghausen I, Konig B, Becker WM, Grabbe S, Petersen A, Saloga J. Gram-positive bacteria on grass pollen exhibit adjuvant activity inducing inflammatory T cell responses. Clin Exp Allergy. 2012; 42:76–84.

57. Abos-Gracia B, del Moral MG, Lopez-Relano J, Viana-Huete V, Castro L, Villalba M, Martinez-Naves E. Olea europaea pollen lipids activate invariant natural killer T cells by upregulating CD1d expression on dendritic cells. J Allergy Clin Immunol. 2013; 131:1393–1399.
58. Brewer JM, Pollock KG, Tetley L, Russell DG. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J Immunol. 2004; 173:6143–6150.

59. Fernandes H, Michalska K, Sikorski M, Jaskolski M. Structural and functional aspects of PR-10 proteins. FEBS J. 2013; 280:1169–1199.

60. Bashir ME, Lui JH, Palnivelu R, Naclerio RM, Preuss D. Pollen lipidomics: lipid profiling exposes a notable diversity in 22 allergenic pollen and potential biomarkers of the allergic immune response. PLoS One. 2013; 8:e57566.

61. Martin-Pedraza L, Gonzalez M, Gomez F, Blanca-Lopez N, Garrido-Arandia M, Rodriguez R, Torres MJ, Blanca M, Villalba M, Mayorga C. Two nonspecific lipid transfer proteins (nsLTPs) from tomato seeds are associated to severe symptoms of tomato-allergic patients. Mol Nutr Food Res. 2016; 60:1172–1182.

62. Kleine-Tebbe J, Hamilton RG. Cashew allergy, 2S albumins, and risk predictions based on IgE antibody levels. Allergy. 2017; 72:515–518.

63. Seutter von Loetzen C, Hoffmann T, Hartl MJ, Schweimer K, Schwab W, Rosch P, Hartl-Spiegelhauer O. Secret of the major birch pollen allergen Bet v 1: identification of the physiological ligand. Biochem J. 2014; 457:379–390.

64. Hilger C, Kuehn A, Hentges F. Animal lipocalin allergens. Curr Allergy Asthma Rep. 2012; 12:438–447.

65. Thomas WR, Hales BJ, Smith WA. Genetically engineered vaccines. Curr Allergy Asthma Rep. 2005; 5:197–203.

66. Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, az-Perales A, Jensen-Jarolim E. The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron. PLoS One. 2014; 9:e104803.

67. Roth-Walter F, Gomez-Casado C, Pacios LF, Mothes-Luksch N, Roth GA, Singer J, az-Perales A, Jensen-Jarolim E. Bet v 1 from birch pollen is a lipocalin-like protein acting as allergen only when devoid of iron by promoting Th2 lymphocytes. J Biol Chem. 2014; 289:17416–17421.

68. Egger M, Hauser M, Mari A, Ferreira F, Gadermaier G. The role of lipid transfer proteins in allergic diseases. Curr Allergy Asthma Rep. 2010; 10:326–335.

69. Yeats TH, Rose JK. The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Sci. 2008; 17:191–198.

70. Agati G, Brunetti C, Di FM, Ferrini F, Pollastri S, Tattini M. Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol Biochem. 2013; 72:35–45.

71. Akkerdaas JH, Schocker F, Vieths S, Versteeg S, Zuidmeer L, Hefle SL, Aalberse RC, Richter K, Ferreira F, van RR. Cloning of oleosin, a putative new hazelnut allergen, using a hazelnut cDNA library. Mol Nutr Food Res. 2006; 50:18–23.

72. Leduc V, Moneret-Vautrin DA, Tzen JT, Morisset M, Guerin L, Kanny G. Identification of oleosins as major allergens in sesame seed allergic patients. Allergy. 2006; 61:349–356.

73. Angelina A, Sirvent S, Palladino C, Vereda A, Cuesta-Herranz J, Eiwegger T, Rodriguez R, Breiteneder H, Villalba M, Palomares O. The lipid interaction capacity of Sin a 2 and Ara h 1, major mustard and peanut allergens of the cupin superfamily, endorses allergenicity. Allergy. 2016; 71:1284–1294.

74. Petersen A, Rennert S, Kull S, Becker WM, Notbohm H, Goldmann T, Jappe U. Roasting and lipid binding provide allergenic and proteolytic stability to the peanut allergen Ara h 8. Biol Chem. 2014; 395:239–250.

75. Salcedo G, Sanchez-Monge R, Barber D, Diaz-Perales A. Plant non-specific lipid transfer proteins: an interface between plant defence and human allergy. Biochim Biophys Acta. 2007; 1771:781–791.

76. Pacios LF, Gomez-Casado C, Tordesillas L, Palacin A, Sanchez-Monge R, Diaz-Perales A. Computational study of ligand binding in lipid transfer proteins: Structures, interfaces, and free energies of protein-lipid complexes. J Comput Chem. 2012; 33:1831–1844.

77. Tordesillas L, Gomez-Casado C, Garrido-Arandia M, Murua-Garcia A, Palacin A, Varela J, Konieczna P, Cuesta-Herranz J, Akdis CA, O'Mahony L, Diaz-Perales A. Transport of Pru p 3 across gastrointestinal epithelium - an essential step towards the induction of food allergy. Clin Exp Allergy. 2013; 43:1374–1383.

78. Yin SC, Liao EC, Chiu CL, Chang CY, Tsai JJ. Der p2 Internalization by epithelium synergistically augments toll-like receptor-mediated proinflammatory signaling. Allergy Asthma Immunol Res. 2015; 7:393–403.

79. Mattila K, Renkonen R. Modelling of Bet v 1 binding to lipids. Scand J Immunol. 2009; 70:116–124.

80. Li J, Wang Y, Tang L, de Villiers WJ, Cohen D, Woodward J, Finkelman FD, Eckhardt ER. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J Allergy Clin Immunol. 2013; 131:442–450.

81. Wang TY, Liu M, Portincasa P, Wang DQ. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest. 2013; 43:1203–1223.

82. Sancho AI, Wangorsch A, Jensen BM, Watson A, Alexeev Y, Johnson PE, Mackie AR, Neubauer A, Reese G, Ballmer-Weber B, Hoffmann-Sommergruber K, Skov PS, Vieths S, Mills EN. Responsiveness of the major birch allergen Bet v 1 scaffold to the gastric environment: impact on structure and allergenic activity. Mol Nutr Food Res. 2011; 55:1690–1699.

83. Vassilopoulou E, Rigby N, Moreno FJ, Zuidmeer L, Akkerdaas J, Tassios I, Papadopoulos NG, Saxoni-Papageorgiou P, van Ree R, Mills C. Effect of in vitro gastric and duodenal digestion on the allergenicity of grape lipid transfer protein. J Allergy Clin Immunol. 2006; 118:473–480.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download