Abstract
The induction of interleukin (IL)-32 in bone marrow (BM) inflammation is crucial in graft versus host disease (GvHD) that is a common side effect of allogeneic BM transplantation. Clinical trials on α-1 antitrypsin (AAT) in patients with GvHD are based on the preliminary human and mouse studies on AAT reducing the severity of GvHD. Proteinase 3 (PR3) is an IL-32-binding protein that was isolated from human urine. IL-32 primarily induces inflammatory cytokines in myeloid cells, probably due to PR3 expression on the membrane of the myeloid lineage cells. The inhibitory activity of AAT on serine proteinases may explain the anti-inflammatory effect of AAT on GvHD. However, the anti-inflammatory activity of AAT on BM cells remains unclear. Mouse BM cells were treated with IL-32γ and different inflammatory stimuli to investigate the anti-inflammatory activity of AAT. Recombinant AAT-Fc fusion protein inhibited IL-32γ-induced IL-6 expression in BM cells, but failed to suppress that induced by other stimuli. In addition, the binding of IL-32γ to PR3 was abrogated by AAT-Fc. The data suggest that the specific anti-inflammatory effect of AAT in mouse BM cells is due to the blocking of IL-32 binding to membrane PR3.
α1-antitrypsin (AAT) exhibits anti-inflammatory activity and its deficiency causes respiratory and inflammatory disorders (12345678). AAT directly blocks serine proteinases activating interleukin (IL)-1 cytokine family, IL-8, IL-32, and tumor necrosis factor (TNF)-α (91011). IL-32, originally discovered as a natural killer cell transcript 4 (NK4) and renamed as IL-32 (also known as TNFα-inducing factor (TAIF) in databank), induces various inflammatory cytokines via NF-κB and p38MAPK signaling (1012131415161718). IL-32α-ligand affinity chromatography lead to isolation of neutrophil proteinase 3 (PR3) from human urine (9). Mature PR3 specifically binds to IL-32 and cleaves it. The suppression of IL-32 expression by AAT increased survival in an allogeneic bone marrow (BM) transplantation mouse model (19). Unlike other cytokines possessing specific membrane receptors, IL-32 utilizes membrane PR3 to induce inflammation. Therefore, this study aimed to investigate the inhibitory activity of AAT on IL-32-induced inflammation in mouse BM cells.
Primary mouse BM cells were isolated from 6-week-old female C57BL/6 mice as described previously (20), seeded on a 96-well plate at a density of 1×106/0.2 mL, and then stimulated with 100 ng/mL LPS (Sigma-Aldrich, St. Louis, MO, USA), TNFα, IL-32γ, and IL-1α (YbdYbiotech, Seoul Korea) as indicated at the bottom. The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University. IL-32γ-induced NF-κB and p38MAPK phosphorylation in BM cells isolated from 6-week-old C57BL/6 mice was examined using anti-phospho antibodies (Cell Signaling Technology, Beverly, MA, USA). Recombinant PR3-Fc was expressed as described previously (21), and then was used in IL-32γ binding assay. A 96-well plate was coated with PR3-Fc (1 µg/mL) overnight and blocked with 3% BSA for 2 h. IL-32γ was added at the concentration indicated at the bottom, and was detected by rabbit anti-IL-32γ antibody (22). AAT-Fc was pre-incubated with PR3-Fc-coated 96-well plate at a concentration indicated at the bottom, and then its reaction with IL-32γ was detected by rabbit anti-IL-32γ antibody.
Here, the anti-inflammatory activity of AAT was investigated in BM cells. Primary mouse BM cells were stimulated with LPS, TNFα, IL-32γ, and IL-1α because they trigger common inflammatory pathways, such as NF-κB and p38MAPK, although they possess a specific receptor. IL-1α induced IL-6 at the highest level, whereas TNFα induced IL-6 at the lowest level, as compared to the induction level of other stimuli (Fig. 1A). Mouse BM cells were pre-incubated with recombinant AAT-Fc and then triggered with the same stimuli (Fig. 1B). Interestingly, AAT-Fc specifically suppressed IL-32γ-induced IL-6 expression, while AAT-Fc failed to inhibit IL-6 expression induced by other stimuli (Fig. 1B). This data is presented for the first time with direct evidence that AAT-Fc inhibits the inflammatory activity of IL-32 in mouse BM cells. In addition, AAT-Fc experiments were performed by pretreating mice for 48 h prior to isolating BM cells. As shown in Fig. 1E, IL-32γ-induced IL-6 expression was further decreased in AAT-Fc-pretreated mice, but IL-6 expression induced by other stimuli was not inhibited (Fig. 1C, D, and F).
Next, NF-κB and p38MAPK signaling were assessed in the mouse BM cells after IL-32γ stimulation. The phosphorylation of NF-κB and p38MAPK was increased by IL-32γ treatment (Fig. 2A), while it was significantly suppressed in the BM cells from AAT-Fc-administered mice (Fig. 2B). In addition, recombinant PR3-Fc was expressed in Chinese Hamster Ovary cells by using the open reading frame of PR3 fused to human Fc as described previously (21). The PR3-Fc protein was used to indicate IL-32γ binding to PR3. IL-32γ binds to PR3-Fc in 1 nM range with a high affinity, since approximately 30 kDa IL-32γ exhibits a specific binding at 30 ng/mL (Fig. 2C). The specific IL-32γ binding to PR3-Fc was abrogated by AAT-Fc in a dose-dependent manner (Fig. 2D).
These data suggest that AAT-Fc blocks IL-32-induced inflammation in BM cells by competing with IL-32γ to bind on membrane PR3 and increases the survival of allogeneic BM transplantation mouse model (Fig. 2E). Unlike other cytokines with specific membrane receptors, IL-32 utilizes membrane PR3 to induce inflammation. This may explain the specific inhibitory activity of AAT on IL-32-induced inflammation in patients with allogeneic BM transplantation. The present study illustrates the function of IL-32 in BM inflammation that is specifically suppressed by AAT-Fc. Further investigation on IL-32-mediated inflammation in human BM transplantation will help understand the causative factor in GvHD.
Figures and Tables
Figure 1
The anti-inflammatory effect of recombinant AAT-Fc in mouse BM cells. (A) IL-6 level in the supernatant of mouse BM cells was measured by sandwich ELISA (R&D Systems, Minneapolis MN). (B) The induction of IL-6 by different stimuli was measured without AAT-Fc (open bar) or with pre-incubation of AAT-Fc (closed bar, 2 µg/mL) for 3 h. (C) LPS-, (D) TNFα-, (E) IL-32γ-, and (F) IL-1α-mediated IL-6 level in the supernatant of BM cells isolated from the mice treated with AAT-Fc for 48 h was compared to that in the supernatant of BM cells isolated from the untreated control mice. Data in A are comparisons between untreated control and stimulated mice. Data in B are comparisons between RPMI control and AAT-Fc pre-incubated cells. Data in E are comparisons between BM cells isolated from untreated mice and BM cells isolated from mice treated with AAT-Fc (2 mg/kg) for 48 h. Mean±SEM; *p<0.05; #p<0.001 from three replicates. Representative data from 1 of 3 independent experiments are shown.
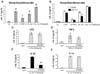
Figure 2
Suppression of inflammatory signaling in recombinant AAT-Fc-pretreated mouse BM cells, AAT-Fc competing with IL-32γ binding to PR3-Fc, and the dogma of AAT anti-inflammatory activity on IL-32-induced inflammation in BM transplantation. (A) The phosphorylation of NF-κB and p38MAPK by IL-32γ in BM cells from 6-week-old C57BL/6 mice was assessed using anti-phospho antibodies. (B) Similar experiments were performed by injecting AAT-Fc (2 mg/kg) into mice for 48 h prior to isolating BM cells. (C) Recombinant PR3-Fc was used for IL-32γ binding assay. IL-32γ was added at the concentration indicated on the bottom, and IL-32γ binds to PR3-Fc in a dose dependent manner. (D) The binding of IL-32γ was blocked with PR3-Fc in a dose dependent manner. Data in C are comparisons between control blank and IL-32γ-treated cells. Data in D are comparisons between control without AAT-Fc and with AAT-Fc. Mean±SEM; *p<0.05; **p<0.01; #p<0.001 from three replicates. Representative data from 1 of 4 independent experiments are shown. (C) Schematic representation of AAT-Fc suppression on IL-32-mediated inflammation in BM transplantation. Allogeneic BM transplantation induces different pro-inflammatory cytokines, such as IL-32, TNFα, IL-1α, and IL-6, resulting in immune reaction causing GvHD and death, while the specific IL-32 blockade AAT-Fc reduces immune reaction increasing survival.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF-2015R1A2A2A01003472, -2014 M3A6A4075058, -2015R1A2A1A15051472), BK21 plus project fund, and this paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2014. This study was supported by Veterinary Science Research Institute of The Konkuk University.
References
1. Schwartz RH, Van Ess JD, Johnstone DE, Dreyfuss EM, Abrishami MA, Chai H. Alpha-1 antitrypsin in childhood asthma. J Allergy Clin Immunol. 1977; 59:31–34.

2. Becker K, Frieling T, Haussinger D. Quantification of fecal alpha 1-antitrypsin excretion for assessment of inflammatory bowel diseases. Eur J Med Res. 1998; 3:65–70.
3. Campbell EJ, Campbell MA, Boukedes SS, Owen CA. Quantum proteolysis by neutrophils: implications for pulmonary emphysema in alpha 1-antitrypsin deficiency. J Clin Invest. 1999; 104:337–344.

4. Elzouki AN, Eriksson S, Lofberg R, Nassberger L, Wieslander J, Lindgren S. The prevalence and clinical significance of alpha 1-antitrypsin deficiency (PiZ) and ANCA specificities (proteinase 3, BPI) in patients with ulcerative colitis. Inflamm Bowel Dis. 1999; 5:246–252.

5. Malerba M, Radaeli A, Ceriani L, Tantucci C, Grassi V. Airway hyperresponsiveness in a large group of subjects with alpha1-antitrypsin deficiency: a cross-sectional controlled study. J Intern Med. 2003; 253:351–358.

6. Aldonyte R, Eriksson S, Piitulainen E, Wallmark A, Janciauskiene S. Analysis of systemic biomarkers in COPD patients. COPD. 2004; 1:155–164.

7. Griese M, Latzin P, Kappler M, Weckerle K, Heinzlmaier T, Bernhardt T, Hartl D. alpha1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J. 2007; 29:240–250.

8. Kwak A, Lee Y, Kim H, Kim S. Intracellular interleukin (IL)-1 family cytokine processing enzyme. Arch Pharm Res. 2016; 39:1556–1564.

9. Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci U S A. 2006; 103:3316–3321.

10. Kim S, Lee S, Her E, Bae S, Choi J, Hong J, Jaekal J, Yoon D, Azam T, Dinarello CA, Kim S. Proteinase 3-processed form of the recombinant IL-32 separate domain. BMB Rep. 2008; 41:814–819.

11. Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J Biol Chem. 2012; 287:8205–8213.

12. Kim SJ, Lee S, Kwak A, Kim E, Jo S, Bae S, Lee Y, Ryoo S, Choi J, Kim S. Interleukin-32gamma transgenic mice resist LPS-mediated septic shock. J Microbiol Biotechnol. 2014; 24:1133–1142.
14. Jhun H, Choi J, Hong J, Lee S, Kwak A, Kim E, Jo S, Ryoo S, Lim Y, Yoon DY, Hong JT, Kim TS, Lee Y, Song K, Kim S. IL-32gamma overexpression accelerates streptozotocin (STZ)-induced type 1 diabetes. Cytokine. 2014; 69:1–5.

15. Bae S, Kim YG, Choi J, Hong J, Lee S, Kang T, Jeon H, Hong K, Kim E, Kwak A, Lee CK, Yoo B, Park YB, Song EY, Kim S. Elevated interleukin-32 expression in granulomatosis with polyangiitis. Rheumatology (Oxford). 2012; 51:1979–1988.

16. Jaekal J, Jhun H, Hong J, Park S, Lee J, Yoon D, Lee S, Her E, Yang Y, G Rho, Kim S. Cloning and characterization of bovine interleukin-32 beta isoform. Vet Immunol Immunopathol. 2010; 137:166–171.

17. Hong J, Bae S, Kang Y, Yoon D, Bai X, Chan ED, Azam T, Dinarello CA, Lee S, Her E, Rho G, Kim S. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 2010; 49:171–176.

18. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005; 22:131–142.
19. Marcondes AM, Li X, Tabellini L, Bartenstein M, Kabacka J, Sale GE, Hansen JA, Dinarello CA, Deeg HJ. Inhibition of IL-32 activation by alpha-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood. 2011; 118:5031–5039.

20. Lee S, Kim E, Jhun H, Hong J, Kwak A, Jo S, Bae S, Lee J, Kim B, Lee J, Youn S, Kim S, Kim M, Kim H, Lee Y, Choi DK, Kim YS, Kim S. Proinsulin shares a motif with interleukin-1alpha (IL-1alpha) and induces inflammatory cytokine via interleukin-1 receptor 1. J Biol Chem. 2016; 291:14620–14627.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download