Abstract
Cancer is one of the leading causes of morbidity and mortality worldwide; therefore there is a need to discover new therapeutic modules with improved efficacy and safety. Immune-(cell) therapy is a promising therapeutic strategy for the treatment of intractable cancers. The effectiveness of certain chemotherapeutics in inducing immunogenic tumor cell death thus promoting cancer eradication has been reported. Ginsenoside Rg3 is a ginseng saponin that has antitumor and immunomodulatory activity. In this study, we treated tumor cells with Rg3 to verify the significance of inducing immunogenic tumor cell death in antitumor therapy, especially in DC-based immunotherapy. Rg3 killed the both immunogenic (B16F10 melanoma cells) and non-immunogenic (LLC: Lewis Lung Carcinoma cells) tumor cells by inducing apoptosis. Surface expression of immunogenic death markers including calreticulin and heat shock proteins and the transcription of relevant genes were increased in the Rg3-dying tumor. Increased calreticulin expression was directly related to the uptake of dying tumor cells by dendritic cells (DCs): the proportion of CRT+ CD11c+ cells was increased in the Rg3-treated group. Interestingly, tumor cells dying by immunogenic cell death secreted IFN-γ, an effector molecule for antitumor activity in T cells. Along with the Rg3-induced suppression of pro-angiogenic (TNF-α) and immunosuppressive cytokine (TGF-β) secretion, IFN-γ production from the Rg3-treated tumor cells may also indicate Rg3 as an effective anticancer immunotherapeutic strategy. The data clearly suggests that Rg3-induced immunogenic tumor cell death due its cytotoxic effect and its ability to induce DC function. This indicates that Rg3 may be an effective immunotherapeutic strategy.
Cancer is one of the leading causes of morbidity and mortality worldwide. Although, cancer progression is mainly driven by the expansion of tumor cells, tumor microenvironment and/or anti-tumor immunity may also play important roles (1). The main treatment options for cancer are surgery, chemotherapy, and radiotherapy however, these methods have serious side effects including toxicity to normal cell and tissue (2). It is thought that cancer cells show immunogenic or non-immunogenic characteristics in their growth e.g. B16F10, a mouse melanoma cell line and LLC, a mouse lung cancer cell line, were shown to have immunogenic and non-immunogenic characteristics, respectively. Immunogenic tumors may respond more sensitively to immunotherapy.
Recent studies have shown the induction of immunogenic tumor cell death by certain chemotherapeutics as promising strategy for cancer therapy. Immunogenic cell death is characterized by the early cell surface exposure of chaperone proteins calreticulin (CRT) and HSPs, which affect DC maturation and the uptake and presentation of tumor antigens by DCs (34567). Thus, inducing immunogenic tumor cell death may enhance the effectiveness of DC-based antitumor therapeutic modules.
Among the saponins originating from plant, ginsenoside is derived from Ginseng, which was originally used in ancient times for the treatment of diseases (89). One of the Ginsenosides, Rg3, has been known to kill tumor cells as well as modulate the immune system (101112). Numerous studies have demonstrated that Rg3 suppresses tumor growth by inhibiting the invasive and metastatic ability of various tumors including lung (1314151617) and ovarian carcinoma cells (18) and/or by promoting the apoptosis of melanoma cells (19). Very few studies have been done into whether the immunomodulatory activity of Rg3 has any anticancer effects, particularly the role of Rg3 in inducing immunogenic tumor cell death.
In this study, we treated tumor cells with ginsenoside Rg3 with the aim of verifying the significance of inducing immunogenic tumor cell death in antitumor therapy, especially in DC-based immunotherapy.
Pathogen-free female C57BL/6 mice, at 5~6 weeks old, were purchased from the Orient Bio (Seongnam, South Korea). The mice were provided with water and food ad libitum and quarantined under a 12 h light: 12 h dark photoperiod in the animal care facility of the Animal Resource Center at the Asan Institute for Life Science and Technology, Asan Medical Center, Seoul, Korea. Animal care was performed following the ILAR guideline. The mice were acclimated for at least one week before any experiments were conducted.
Ginsenoside Rg3 was supplied by Dr. Sung Ho Son (VitroSys Inc., Yeongju, Korea). Doxorubicin hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM) and gentamicin were obtained from GIBCO laboratories (Grand Island, NY, USA) and fetal bovine serum (FBS) was obtained HyClone Laboratories (Logan, UT, USA). The following antibodies for flow cytometric phenotyping were purchased from eBioscience (SanDiego, CA, USA); fluorescence labeled-monoclonal Abs against CRT, HSP60, HSP70, HSP90, and Annexin V/PI. ELISA kits for cytokines including IFN-γ, IL-6, TGF-β1, and TNF-α were purchased from eBioscience (SanDiego, CA, USA).
C57BL/6 syngeneic Lewis Lung Carcinoma (LLC) and B16F10 (melanoma) cell lines were purchased from the American Type Culture Collection (ATCC) (Rockville, MD, USA). All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 10 mg/ml gentamicin.
The Cell Counting Kit-8 (CCK-8, Dojindo Labratories, Kumamoto, Japan) was used to measure the cytotoxicity of Rg3 on LLC and B16F10 cells. The cells (2×103 cells/well) were cultured in the presence of Rg3 (100, 200, 300, 400, 500 µg/ml) for 48 h in 96-well plates at 37℃ in a 5% CO2 incubator. After 10 µl CCK-8 solution was added to each well, the plate was re-incubated for 3 h at 37℃ and the absorbance at 450 nm was detected using a microplate reader (µ Quant MQX200; Bio-Tek, Winooski, Vermont, USA).
The phenotype and ability to induce immunogenicity of Rg3-treated cells (LLC, B16F10) were analyzed by direct immunofluorescence staining of cell surface antigens using fluorescein isothiocyanate (FITC)-or phycoerythrin (PE) conjugated antibodies against HSP60, HSP70, HSP90, and CRT. Single cells were incubated with fluorescence-labeled surface antibodies in PBS with 0.1% sodium azide and 1% FBS (PBS-CS) for 40 min at 4℃. No more than 1 h after antibody labeling, cells in the 300 µl PBS-CS analyzed with CytoFLEX (Beckman-Coulter, Pasadena, CA, USA). Data was analyzed using Flowjo Software (LLC, Ashland, OR, USA).
Cell death was assessed by Annexin V-fluorescein isothiocyanate staining. Cells were collected, washed in PBS, and resuspended in an incubation buffer containing Annexin V-fluorescein isothiocyanate antibodies. The samples were kept in the dark and incubated for 15 minutes before the addition of 0.1% propidium iodide (PI). They were then analyzed on a CytoFLEX (Beckman-Coulter, Pasadena, CA, USA). Data were analyzed using Flowjo Software (LLC, Ashland, OR, USA).
The concentration of cytokines secreted into the Rg3-treated tumor cell culture supernatants, were measured using commercial ELISA kits for IFN-γ, IL-6, TGF-β1, and TNF-α (eBioscience, SanDiego, CA, USA).
Total RNA was isolated, labeled, and prepared for hybridization to an 11K mouse oligonucleotide microarray gene chip (Macrogen Inc., Seoul, Korea) following the manufacturer's instructions. Hybridization was then conducted overnight using 15 µg of labeled RNA product, after which the arrays were scanned using Affymetrix scanners. The gene expression profile of the cells were created using the Affymetrix system (Beyond Bioinformatics ISTECH AATC Gyeonggi, South Korea) in conjunction with the Mouse Genome 430A 2.0 Array, which contains approximately 54675 probes. Pre-treatment was conducted using the GCOS global scaling in GenPlex software (Istech Corp., Korea). Differences in the distribution of data were confirmed by comparing an MA plot of the control array to a plot of the experimental array. Data were considered significant when gene expression changed by at least two-fold at three consecutive time-points when compared to the expression of the control (0 h). Increased gene expression also had to include at least one present call (Affymetrix algorithm) or both control points needed to be present when gene expression increased or decreased.
To evaluate the cytotoxicity of Rg3 against the LLC, non-immunogenic tumor cells and B16F10, immunogenic tumor cells, CCK-8 assay was performed using different concentrations of Rg3 (10, 20, 50, 100, 200, 300, 400, 500 µg/ml) for 48 h. As shown in Fig. 1A and B, Rg3 kill both LLC and B16F10 cells in a dose-dependent manner. B16F10 cells were more sensitive to Rg3-induced cell death than LLC. Although much weaker than doxorubicin, a well-known chemotherapeutic agent, this data confirms the direct tumor cell cytotoxicity of Rg3.
Assays were employed to investigate the type of cell death induced by Rg3. Rg3 induced apoptosis rather than necrosis. Rg3 induced the early (Annexin V+/PI−) and late (Annexin V+/PI+ ) stages of apoptosis in LLC and B16F10 tumor cells in a dose-dependent manner (Fig. 2). At a dose that evoked similar cell death (Fig. 1 Doxo 0.01 µg/ml, Rg3 500 µg/ml), this phenomenon was differentiated from Doxorubicin induced death, which was mainly necrotic (Annexin V−/PI+ ).
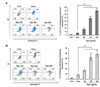
Surface expression of immunogenicity-inducing molecules on the tumor cells killed by Rg3: To observe the ability of Rg3 to induce immunogenic cell death, tumor cell surface molecular expressions were analyzed by flow cytometry. Significant expression of HSP60, HSP70, HSP90, and CRT on LLC and B16F10 cells was detected 18 hours after treatment with a 300 µg/ml dose (~IC50) of Rg3 (Fig. 3A and B). These findings suggest that Rg3-treated tumor cells may be more easily taken up and presented to the immune system by DC, when compared to control cells. After Rg3 treatment, CRT expression increased more in B16F10 cells than in LLC cells. On the other hand, in LLC cells, higher levels of HSP were expressed when compared to B16F10 cells.
Cytokine secretion from the Rg3 treated LLC and B16F10 cells: The most significant finding was that the secretion of IFN-γ from both tumor cell lines was induced by Rg3 treatment in dose-dependent manner (Fig. 4A and B). The secretion of inflammatory cytokines IL-6, TNF-α, and TGF-β1 from the Rg3-treated tumor cells were significantly reduced.
Splenic DCs (CD11c+) were co-cultured with tumor cells pre-labeled with CRT for 18 h and, using FACS, we analyzed the uptake of tumor cells by DCs (Fig. 5A and B). Both LLC and B16F10 tumor cells expressed HSPs and CRT on their surface after Rg3 treatment (Fig. 3A and B). To confirm the role of CRT as an 'eat-me' signal, DC uptake of Rg3-treated tumor cells expressing CRT was observed. The proportion of CRT+ CD11c+ double positive cells in the co-culture of Rg3-treated tumor cell and CD11c+ DCs was measured. Rg3 treatment clearly induced DC uptake of LLC (15.3% vs. 39% with 0 or 300 µg/ml Rg3 respectively) and B16F10 (7.72% vs. 52.8% with 0 or 300 µg/ml Rg3 respectively) tumor cells.
To observe the differential gene expression related to Rg3-induced immunogenic cell death, mRNA microarray analysis was performed (Fig. 6A and B). The gene transcription of CRT (CALR), Hsp60, and HMGB1 which are related to the immunogenicity induction of dead cells, was upregulated in the Rg3-treated tumor cells. On the other hand, VEGF (VEGFA) and TNF (TNFRSF9) receptor gene levels were downregulated by Rg3 treatment. As was expected, the transcription of apoptosis related genes (ATG5, BCL2L13) were upregulated by Rg3 treatment.
Ginsenoside Rg3, one of the ginseng saponins, has been reported to possess a potent antitumor and immunomodulatory activity. Both LLC lung cancer cells and B16F10 melanoma cells were killed by high doses of Rg3 (over 100 µg/ml) in a dose-dependent manner. Melanoma cells were more sensitive to Rg3-induced cytotoxicity than lung cancer cells. Nonetheless, the direct cytotoxicity of Rg3 to tumor cells is not strong enough to be compared to known chemotherapeutics like doxorubicin.
Along with its direct cytotoxicity, the immunomodulatory activity of Rg3 has been observed as a mechanism of antitumor effect. Thus, the possibility of immunogenic tumor cell death induced by Rg3 has been studied. Immunogenic cell death is characterized by the early cell surface exposure of chaperone proteins including CRT and HSPs, which affect DC maturation and the uptake and presentation of tumor antigens. Thus, the induction of immunogenic tumor cell death may increase the efficacy of antitumor immunotherapy including DC-based therapeutic cancer vaccines. Immunogenic cell death is initiated by the induction of apoptosis. Translocation of CRT or HSPs onto the dying cell surface is considered as a molecular mechanism underlying the increased immunogenicity of apoptotic tumor cells. In this study, Rg3-mediated induction of apoptotic tumor cell death and a subsequent increase in the expression of CRT and HSPs on the surface of dying lung cancer (LLC) and melanoma cells (B16F10) were observed. Induction of apoptosis and increased surface protein expression of CRT or Hsp60 on the Rg3-induced dying tumor cells were supported by an increase in the transcription of related apoptotic genes (BCL2L13, an apoptosis facilitator / CALR or HSP60 for CRT or Hsp60, respectively) as observed using mRNA microarray analysis. Another known marker indicating immunogenic cell death, HMGB1, showed increased gene transcription (2~4 times) in the Rg3-induced dying tumor cells of both LLC and B16F10, when compared to the control. The role of CRT as an 'eat-me' signal, presented to DCs by dying tumor cells to signal their uptake, was confirmed by the co-culture of Rg3-treated tumor cell with DCs. DCs took up the Rg3-treated tumor cells more readily than control cells. This was observed by the increased proportion of CD11c (DC marker) and CRT double positive cells using flow cytometry.
An interesting phenomenon related to the Rg3-induced immunogenic tumor cell death was the secretion of IFN-γ from Rg3-treated tumor cells. IFN-γ is known to be an antitumor effector molecule produced by T cells not by tumor cells. However, our previous study (20) reported that MC38 colon cancer cells killed by chemically inducing immunogenic cell death secreted IFN-γ. In addition, overexpression of IFN-γ in B16F10 cells inhibited tumor growth and induced apoptotic cell death (21). Along with the Rg3-induced suppression of pro-angiogenic (TNF-α) or immune-suppressive cytokine (TGF-β) secretion, IFN-γ production from the Rg3-treated tumor cells also indicates that Rg3 may be an effective anticancer immunotherapeutic module.
Although Rg3 induced the immunogenic cell death of both LLC, Lewis Lung Carcinoma and B16F10, melanoma cells, each parameter for cell death, surface molecule including CRT expression and cytokine secretion was differentially modulated. B16F10 melanoma cells are known to be immunogenic and were very sensitive to Rg3-induced cell death. However, compared to LLC, the cell death induced by Rg3 in B16F10 was more like necrosis. Surface expression of CRT was similar in the two tumor cell lines, but the expression of HSPs was higher in LLC, a typical non-immunogenic tumor cells. There were also a greater proportion of Rg3-treated melanoma cells taken up by DC in comparison to lung cancer cells.
Our data suggests that Rg3 induced immunogenic tumor cell death in not only immunogenic tumor like melanoma but also in non-immunogenic tumor like lung cancer cells. This ability of Rg3 confers an advantage in using it as an immunotherapeutic module. Most importantly, Rg3-induced cell death can result in the conversion of non-immunogenic to immunogenic tumor cells. Thus, normally intractable non-immunogenic tumor cells like lung cancer cells could respond sensitively to antitumor immunity such as DC uptake.
Figures and Tables
 | Figure 1The effect of ginsenoside Rg3 treatment on the viability of LLC and B16F10 cells. The LLC (A) and B16F10 (B) cells (2×103 cells/well) were seeded in 96-well plates and treated with different Rg3 concentrations (10~500 µg/ml) and Doxorubicin (0.01 µg/ml) for 48 h. Each value is the mean±SE of three experiments. Each experiment was done in triplicate. Significant effects compared to control are indicated with asterisks (*p<0.05 or **p<0.01). |
 | Figure 2Apoptotic cell death was observed in LLC (A) and B16F10 (B) cell lines. Cells were treated for 18 h with the indicated Rg3 (100, 300, 500 µg/ml) and Doxorubicin (0.01 µg/ml) concentrations. The percentage of apoptotic cells were determined by measuring Annexin V/PI expression using flow cytometry. Each experiment was done in triplicate. Significant effects versus control are indicated with asterisks (*p<0.05, **p<0.01); # indicates the significant changes (#p<0.05) compared to Doxorubicin (0.01µg/ml). |
 | Figure 3Rg3 induced the expression of CRT and HSPs on LLC (A) and B16F10 (B) cell lines. Representative histograms of one of the experiments and mean±SE of three experiments showing the expression of CRT, HSP60, HSP70, and HSP90 after 18 h of Rg3 treatment on tumor cells are shown. Each experiment was done in triplicate. Significant effects versus control are indicated with asterisks (*p<0.05, **p<0.01, ***p<0.001); #,##indicates the significant changes (#p<0.05 or ##p<0.01) compared to Doxorubicin (0.01 µg/ml). |
 | Figure 4(A) The effect of ginsenoside Rg3 (100, 300, 500 µg/ml) on cytokine secretion from LLC cells. Each value is the mean±SE of three experiments. Each experiment was done in triplicate. Significant effects versus control are indicated with asterisks (*p<0.05, **p<0.01, ***p<0.001); #indicate the significant changes compared to Doxorubicin (0.01 µg/ml). (B) The effect of ginsenoside Rg3 (100, 300, 500 µg/ml) on cytokine secretion from B16F10 cells. Each value is the mean±SE of three experiments. Each experiment was done in triplicate. Significant effects versus control are indicated with asterisks (*p<0.05, **p<0.01, ***p<0.001); #indicates the significant changes compared to Doxorubicin (0.01 µg/ml). |
 | Figure 5(A) LLC was incubated overnight with or without doxorubicin or Rg3 at 37℃. LLCs were then stained with CRT-FITC. DCs were labeled with CD11c-PerCP5.5 then co-cultured with CRT-FITC labeled LLC for 6 h. FITC and PerCP5.5 double stained cells were considered as DCs taken tumor cells. Each experiment was done in triplicate. (B) B16F10 was incubated overnight with or without doxorubicin or Rg3 at 37℃. B16F10s were then stained with CRT-FITC. DCs were labeled with CD11c-PerCP5.5 then co-cultured with CRT-FITC labeled B16F10 for 6 h. FITC and PerCP5.5 double stained cells were considered as DCs taken tumor cells. Each experiment was done in triplicate. Significant effects versus control are indicated with asterisks (*p<0.05); #indicates the significant changes (#p<0.05) compared to Doxorubicin (0.01µg/ml). |
 | Figure 6(A) Clustering of gene expression in LLC after treatment with Doxorubicin or Rg3. 11 genes were selected based on the fold change in expression (log2). Then, these genes were divided into control. The graph produced showed clusters using the Genplex software and the expression levels of genes are shown as graphs. (B) Clustering of gene expression in B16F10 after treatment with Doxorubicin or Rg3. 11 genes were selected based on the fold change in expression (log2). Then, these genes were divided into control. The graph produced showed clusters using the Genplex software and the expression levels of genes are shown as graphs. |
ACKNOWLEDGEMENTS
The authors sincerely thank to Dr. Sung Ho Son at the Vitrosys Inc., Yeongju, Korea for providing ginsenoside Rg3. This study was supported by the grant from the National Research Foundation of Korea (#2014-055-842).
References
1. Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM, Lee BY, Kwon SM. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012; 13:504–515.

2. Nam KY, Choi JE, Hong SC, Pyo KM, Park JD. Recent Progress in Research on Anticancer Activities of Ginsenoside-Rg3. Korean J Pharmacogn. 2014; 45:1–10.
3. Cirone M, Di RL, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Activation of dendritic cells by tumor cell death. Oncoimmunology. 2012; 1:1218–1219.

4. Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011; 71:4821–4833.

5. Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010; 16:3100–3104.

6. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van EP, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009; 28:578–590.

7. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van EP, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007; 13:54–61.

8. Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997; 60:52–60.

9. Joo EJ, Ha YW, Shin H, Son SH, Kim YS. Generation and characterization of monoclonal antibody to ginsenoside rg3. Biol Pharm Bull. 2009; 32:548–552.

10. Gao JL, Lv GY, He BC, Zhang BQ, Zhang H, Wang N, Wang CZ, Du W, Yuan CS, He TC. Ginseng saponin metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncol Rep. 2013; 30:292–298.

11. Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007; 73:669–674.

12. Liu TG, Huang Y, Cui DD, Huang XB, Mao SH, Ji LL, Song HB, Yi C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009; 9:250.

13. Zhang Q, Kang X, Zhao W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Commun. 2006; 342:824–828.

14. Yi C, Huang XB, Hou M. [Experimental study on effect of chemotherapy combined ginsengnoside Rg3 in treating pulmonary carcinoma]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005; 25:58–59.
15. Hu SS, Zhou LK, Ba Y, Li HI, Zhu CH. A meta-analysis of Ginsenoside Rg3 for non-small cell lung cancer. Clin Oncol Cancer Res. 2011; 8:175–180.

16. Liu JW, Chen JX, Yu LH, Tian YX, Cui XY, Yan Q, Fu L. [Inhibitory effect of ginsenoside-Rg3 on lung metastasis of mouse melanoma transfected with ribonuclease inhibitor]. Zhonghua Zhong Liu Za Zhi. 2004; 26:722–725.
17. Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su MM, Wang DD, Wang WJ. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl). 2008; 121:1394–1397.

18. Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K, Kim TJ, Ham J, Jang HJ, Kang KS, Ko H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014; 322:23–33.

19. Lee SG, Kim BS, Nam JO. Ginsenoside Rg3 induces apoptosis in B16F10 melonoma cells. J Life Sci. 2014; 24:1001–1005.
20. Oh SJ, Ryu CK, Baek SY, Lee H. Cellular mechanism of newly synthesized indoledione derivative-induced immunological death of tumor cell. Immune Netw. 2011; 11:383–389.

21. Park D, Bae DK, Jeon JH, Lee J, Oh N, Yang G, Yang YH, Kim TK, Song J, Lee SH, Song BS, Jeon TH, Kang SJ, Joo SS, Kim SU, Kim YB. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011; 31:397–405.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download