Abstract
K/BxN serum can induce arthritis in normal mice because of abundant autoantibodies that trigger an innate inflammatory response in joints. To determine whether IL-17 is involved in the pathogenesis of serum-induced arthritis, we injected wild-type and IL-17−/− mice with K/BxN serum and evaluated them for signs of arthritis. Unlike wild-type mice, IL-17−/− mice did not show any signs of arthritis. IL-17 was produced predominantly by CD3− CD4− γδTCR− NK1.1− Sca1int Thy1hi cells residing in the inflamed synovial tissue. When synovial cells extracted from normal joints were stimulated with IL-23 or autoantibody-containing immune complexes, a substantial fraction of Sca1int Thy1hi cells produced IL-17. Thus, we have identified a novel population of IL-17-producing innate synovial cells that play a crucial role in the development of K/BxN serum-induced arthritis.
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that primarily affects the synovial membranes of diarthrodial joints (1). As a result of the breakdown of self-tolerance, autoantibodies produced during the initiation phase are deposited into the synovial tissue. During the effector phase, these autoantibodies orchestrate diverse innate immune cells such as mast cells, neutrophils, and synovial fibroblasts to trigger an inflammatory response, leading to progressive destruction of cartilage and bone (2). These pathogenic processes are mirrored relatively well in the K/BxN mouse model.
K/BxN mice spontaneously develop severe arthritis because of KRN transgenic T cells that specifically recognize a self-peptide derived from glucose-6-phosphate isomerase (GPI) in the context of MHC I-Ag7 and anti-GPI autoantibodies (3). Passive transfer of K/BxN serum containing anti-GPI autoantibodies to normal mice can bypass the initiation phase and directly execute effector functioning (4). Therefore, a K/BxN serum transfer model allows for studies that focus specifically on the effector phase, driven mainly by innate immune cells, of inflammatory arthritis.
Neutrophils are abundant in the synovial tissue of RA patients and indispensable for the development of K/BxN serum-induced arthritis (56). Components of signaling networks that chemoattract neutrophils include IL-17. IL-17 is produced by various cells such as Th17 and γδ T cells and innate lymphoid cells, and it elicits IL-17R-expressing cells to produce proinflammatory mediators, including the neutrophil chemoattractant IL-8 (7). The observation that patients with RA have higher levels of IL-17 in their sera and synovial fluids than healthy controls suggests that IL-17 plays a role in the pathogenesis of arthritis (89). In agreement with this, IL-17-deficient mice were found refractory to collagen-induced arthritis (CIA), indicating that IL-17 was essential in this model (10). However, the sources and actions of IL-17 are not fully understood. For example, although Th17 cells and γδ T cells are the main sources of IL-17 in the context of CIA, only Th17 cells, not γδ T cells, induce arthritic bone destruction (11). Moreover, IL-17-producing γδ T cells evident in the CIA model are not present in SKG mice and RA patients (12). In the K/BxN model, IL-17 is specifically required for the generation of anti-GPI autoantibodies (13). However, it is not clear whether IL-17 is also required for the effector phase, since previous studies using the K/BxN serum-induced arthritis model have provided conflicting results (1314).
In this study, we evaluated whether IL-17 is essential for the effector phase of inflammatory arthritis in the K/BxN serum transfer model. Moreover, we identified a novel cell population in the affected synovium that produced IL-17 in response to IL-23 or immune complexes.
IL-17−/− congenic C57BL/6 mice (hereafter referred to as IL-17−/− mice) originally provided by Dr. Iwakura from Tokyo University were bred and maintained under specific pathogen-free conditions in accredited animal facilities at Hanyang University. C57BL/6 mice were purchased from Orient Bio. K/BxN mice were obtained by crossing KRN TCR transgenic mice on a C57BL/6 background (K/B) with NOD mice (15). The study protocol was approved by the Institutional Animal Care and Use Committee of Hanyang University. All animal experiments were carried out in accordance with the committee guidelines and regulations
Serum was collected from 8~12-week-old arthritic K/BxN mice. Wild-type (WT) and IL-17−/− mice at 7 weeks of age were injected intraperitoneally with K/BxN serum (150 µl/mouse). Arthritic signs were evaluated in a blinded manner, and disease severity was assessed using a previously described scoring system (15). Ankle thickness of both hind paws was measured axially across the malleoli using a caliper. Hind paws were removed on day 12 after serum transfer, fixed, and decalcified in 5.5% EDTA in phosphate-buffered formalin. The specimens were embedded in paraffin, sectioned, and stained with H&E.
Hind paw tissues were fixed, embedded in paraffin, and sectioned at 7 mm in thickness. Standard immunohistochemical methods were then applied. Goat rabbit anti-mouse IL-17 Ab (eBioscience, San Diego, CA, USA) was used at the appropriate dilution. The sections were incubated with secondary Ab and exposed to avidin-biotin-peroxidase complexes and 3.3′-diminobenzidine (all from Vector Laboratories, Burlingame, CA, USA), followed by counterstaining with 1% methyl green solution.
To prepare single cell suspensions from synovial cells of the mice, the synovial tissues around ankle joints were collected, sliced into small pieces, digested with 50 µg/ml Liberase (Roche, Switzerland) at 37℃ for 40 min, and then filtered through cell strainers with a 70-µm pore size. The synovial cells were cultured in RPMI 1640 (Welgene, Gyeongsangbuk-do, South Korea) medium containing 10% FBS (Gibco, Detroit, MI, USA) in the presence or absence of 10 ng/ml IL-23 (BD Biosciences, San Jose, CA, USA) or immune complexes for 48 h. Immune complexes were prepared as described previously (16). The cells were stimulated with a mixture of 40 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA), 1 µg/ml ionomycin (Sigma-Aldrich), and Golgi-Stop (BD Biosciences) for the last 5 h of incubation to detect IL-17 expression by FACS.
IL-17 expression by synovial cells was detected by FACS as previously described (17). mAbs to mouse IL-17, Sca1, Thy1, CD11b, CD11c, Gr-1, NK1.1, and c-Kit were purchased from eBioscience, and mAbs to mouse CD4 and γδ TCR were obtained from BD Biosciences.
RNA was purified from synovial cells using TRIzol (Invitrogen, Carlsbad, CA, USA) and assayed by RT-PCR as described previously (18). The following primer sequences were used: IL-17, 5′-TCC AGA AGG CCC TCA GAC TA-3′ and 5′-AGC ATC TTC TCG ACC CTG AA-3′; IL-23, 5′-ATC CAG TGT GAA GAT GGT TG-3′ and 5′-GGA GTT GGC TGA GTC CTA GT-3′; β2 microglobulin (β2M), 5′-TGA CCAGCT TGT ATG CTA TC-3′ and 5′-CAG TGT GAG CCA GGA TAT AG-3′.
Autoantibodies abundant in K/BxN serum can induce arthritis in normal mice by initiating innate inflammatory responses in joints (4). To determine the role of IL-17 in the innate effector phase of arthritis, we assessed the susceptibility of IL-17-deficient mice to K/BxN serum-induced arthritis. Whereas all WT mice developed severe arthritis, evident in ankle thicknesses and arthritic indices, in response to the serum, none of the IL-17−/− mice showed any symptoms of arthritis (Fig. 1A). Accordingly, severe leukocyte infiltration and bone invasion were observed in the hind-paw joints of serum-recipient WT mice, whereas the joint architecture of IL-17−/− mice was intact compared to that of untreated mice (Fig. 1B). IL-17-expressing cells were detected in the hind-paw synovial tissue of serum-recipient WT mice, but not in that of the serum-recipient IL-17−/− mice (Fig. 1C). In addition, IL-17 transcripts were more abundant in the synovial tissue of WT mice after serum transfer, but were undetectable in the tissue from IL-17−/− mice, as expected (Fig. 1D). IL-23 transcripts were similarly increased in both strains of mice in response to serum transfer.
These results indicate that IL-17 is required for the development of arthritis triggered by K/BxN serum and suggest that autoantibodies promote IL-23 expression. IL-23, in turn, activates cells that are residing in or recruited to the synovial tissue to produce IL-17, an essential player in synovitis.
Previous studies have shown that IL-17 is produced not only by CD4+ Th17 cells but also by other types of cells such as γδ TCR+ T cells, iNKT cells, NK cells, mast cells, and innate lymphoid cells with diverse phenotypes (1119202122). To identify the IL-17-producing cells that mediated the K/BxN serum-induced arthritis, we extracted synovial cells from swollen synovial tissues when clinical signs reached their peak and assayed the tissues by FACS. We observed that a small but substantial fraction of IL-17-producing cells were present in the synovial cell subpopulation with higher FSC and SSC levels (hereafter referred to as FSCintSSCint cells), but not in the subpopulation with low FSC and SSC levels. The IL-17-producing cells lacked lineage markers specific for B220+ B cells, CD3+ T cells, CD4+ Th cells, γδ TCR+ T cells, CD11b+ myeloid cells, CD11c+ dendritic cells, Gr-1+ granulocytes, NK1.1+ NK and NKT cells, and c-Kit+ mast cells (Fig. 2A). The IL-17-producing cells were identified in a fraction of Thy1hiSca1int cells within the FSCintSSCint subpopulation (Fig. 2B). Despite the existence in both strains of mice even at the steady state, the Thy1hiSca1int cells produced IL-17 only in WT mice, not in IL-17−/− mice, in response to serum transfer (Fig. 2C). Therefore, these results suggest that IL-17 was produced by cells resident in the synovial tissue, rather than by cell infiltrates in response to arthritogenic stimulation, and these cells retained a phenotype distinct from all other lineages known to express IL-17 in the synovium.
Because a certain population of innate lymphoid cells residing in the intestinal mucosa was recognized to produce IL-17 in response to IL-23 (22), we examined whether IL-17 expression by Thy1hiSca1int synovial cells could be induced by IL-23. To this end, synovial cells extracted from normal mice were stimulated with IL-23, and their IL-17 production was assayed by RT-PCR and FACS. IL-23 treatment greatly increased the level of IL-17 transcripts, as well as the proportion of IL-17-expressing cells within Thy1hiSca1int synovial cells (Fig. 3A).
Because autoantibodies present in K/BxN serum were the major arthritogenic factor, we evaluated whether autoantibody-containing immune complexes could activate Thy1hiSca1int synovial cells to produce IL-17. For this purpose, we generated K/BxN immune complexes by incubating K/BxN serum with GPI protein. A mixture of BxN serum and GPI protein (BxN immune complexes) was used as a negative control. Treatment of synovial cells with K/BxN immune complexes resulted in significantly enhanced IL-17 production by Thy1hiSca1int synovial cells, whereas the effect of BxN immune complexes was marginal (Fig. 3B).
These results, taken together, demonstrate that IL-23 and K/BxN immune complexes promote IL-17 production by Thy1hiSca1int synovial cells in vitro, although it is not clear whether IL-23 and K/BxN immune complexes act directly on Thy1hiSca1int synovial cells or elicit other synovium-resident cells to activate Thy1hiSca1int synovial cells. Interestingly, these cells share characteristics such as the Thy1+Sca1+ phenotype and responsiveness to IL-23 with IL-17-producing innate lymphoid cells identified in inflamed intestines.
Although previous studies have identified diverse populations of innate lymphoid cells, mostly residing in barrier tissues such as the skin, intestine, and lungs (23), there have been no reports of these cells residing in synovial tissues. Here, we provide evidence of IL-17-producing synovium-resident cells. We also revealed that these cells are responsive to immune complexes and IL-23 and thereby play a prominent role in inflammatory arthritis.
Figures and Tables
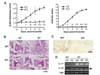 | Figure 1IL-17 is required for development of K/BxN serum-induced arthritis. IL-17−/− mice and their WT littermates were injected with K/BxN serum, disease was assessed for 12 days, and then mice were sacrificed for ex vivo assays. (A) Ankle thickness of hind-paws and arthritis index (n=15 per group). (B) Histopathologic examination of hind-paw sections. Original magnification, 100×. (C) Immunohistochemical staining of hind-paw sections with anti-IL-17 Ab. Original magnification, 200×. (D) Synovial tissues were assayed by RT-PCR. The arrow indicates IL-23. Data shown are pooled (A) or representative (B-D) results of three independent experiments. Graphs display means±SEMs. *p<0.05, **p<0.01, and ***p< 0.001 by Student's t-test. NT, no treatment; ST, serum transfer. |
 | Figure 2Phenotypes of IL-17-producing synovial cells. IL-17−/− mice and their WT littermates were administered K/BxN serum. Synovial cells were extracted from mice post-mortem (day 12 post-serum transfer) and analyzed by FACS. (A) FACS profiles of cells from WT mice, gated on live lymphocytes (R1) or FSCintSSCint cells (R2). (B) The FSCintSSCint cells were divided into three fractions, ScahiThy1hi (R3), Sca1intThy1hi (R4), and remaining (R5) cells, and the percentage of IL-17+ cells within each fraction is shown. (C) FACS profiles displaying Sca1 and Thy1 expression levels of R2-gated cells (the upper panel), and the percentage of IL-17+ cells within the R6 gate of upper graphs (the lower panel). Data are representative of three independent experiments. NT, no treatment; ST, serum transfer. |
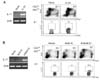 | Figure 3IL-23 and immune complexes promote IL-17 expression by Sca1intThy1hi synovial cells. Synovial cells extracted from normal mice were stimulated with 10 ng/ml IL-23 (A) or immune complexes (IC) (B) for 48 h, followed by RT-PCR and FACS analyses. FACS profiles gated on FSCintSSCint (upper panels) and R1 (lower panels) are shown. Data are representative of three independent experiments. |
ACKNOWLEDGEMENTS
This work was supported by a NRF grant funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A2A1A11052070 awarded to J. Youn).
References
2. Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004; 82:217–248.

3. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996; 87:811–822.

4. Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999; 10:451–461.

5. Brennan FM, Maini RN, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998; 20:133–147.

6. Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001; 167:1601–1608.

7. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010; 10:479–489.

8. Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999; 42:963–970.

9. Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999; 103:1345–1352.

10. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003; 171:6173–6177.

11. Pollinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di PF, O'Reilly T, Horwood NJ, Patel DD, Littlewood-Evans A. Th17 cells, not IL-17+ gammadelta T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011; 186:2602–2612.
12. Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N, Hashimoto M, Sakaguchi N, Sakaguchi S, Yoshifuji H, Nojima T, Ohmura K, Fujii T, Mimori T. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009; 60:2294–2303.

13. Jacobs JP, Wu HJ, Benoist C, Mathis D. IL-17-producing T cells can augment autoantibody-induced arthritis. Proc Natl Acad Sci U S A. 2009; 106:21789–21794.

14. Sadik CD, Kim ND, Alekseeva E, Luster AD. IL-17RA signaling amplifies antibody-induced arthritis. PLoS One. 2011; 6:e26342.

15. Jang E, Kim HR, Cho SH, Paik DJ, Kim JM, Lee SK, Youn J. Prevention of spontaneous arthritis by inhibiting homeostatic expansion of autoreactive CD4+ T cells in the K/BxN mouse mode. Arthritis Rheum. 2006; 54:492–498.

16. Jang E, Cho WS, Cho ML, Park HJ, Oh HJ, Kang SM, Paik DJ, Youn J. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J Immunol. 2011; 186:1546–1553.

17. Lee H, Jin BE, Jang E, Lee AR, Han DS, Kim HY, Youn J. Gut-residing microbes alter the host susceptibility to autoantibody-mediated arthritis. Immune Netw. 2014; 14:38–44.

18. Oh YK, Jang E, Paik DJ, Youn J. Early growth response-1 plays a non-redundant role in the differentiation of B cells into plasma cells. Immune Netw. 2015; 15:161–166.

19. Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007; 178:4466–4472.

20. Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008; 205:1381–1393.

21. Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008; 180:5167–5171.

22. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010; 464:1371–1375.

23. A versatile cell population. Nat Immunol. 2016; 17:754.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download