Abstract
Immune cells (leukocytes or white blood cells) move actively and sensitively based on body conditions. Despite their important role as protectors inside the body, it is difficult to directly observe the spatiotemporal momentum of leukocytes. With advances in imaging technology, the introduction of two-photon microscopy has enabled researchers to look deeper inside tissues in a three-dimensional manner. In observations of immune cell movement along the blood vessel, vascular permeability and innate immune cell movements remain unclear. Here, we describe the neutrophil extravasation cascade, which were observed using a two-photon intravital imaging technique. We also provide evidence for novel mechanisms such as neutrophil body extension and microparticle formation as well as their biological roles during migration.
In single-photon confocal microscopy, a photon is excited and then emitted for visualization of the fluorescent cell or tissue. In the process, fluorescence is emitted along the whole path of a laser beam focused on a sample of fluorescent dye, forming a long trail of light (1). Two-photon microscopy, however, doubles the energy of two near-infrared photons that are absorbed simultaneously (2). Indeed, the light ranges from 700 to 1000 nm (3). As two-photon microscopy uses photons with longer wavelengths and less energy than single-photon confocal microscopy, this results in deep penetration of the photon into the tissue with less tissue damage. This mechanism also enables researchers to visualize only the focal point of a laser beam within a range of 1 µm above and below the focal point (1) and to capture images for much longer periods of time compared to single-photon confocal microscopy.
Confocal fluorescent microscopy has been widely used over the past several decades to observe the morphology and motility of cells and tissues. Many valuable discoveries such as imaging of protein interactions and the analysis of gene expression in living animals (4) have been made using single-photon confocal microscopy. Also, several research groups introduced novel phenomena of immune response from intravital imaging using single-photon confocal microscopy (56). Yet, this microscopic system contains three major limitations: inability to observe ‘deep’ into biological tissues, damage to nearby tissues from photobleaching, and loss of image contrast (2). Thus, single-photon confocal microscopy is more widely used for in vitro or ex vivo imaging of target tissues for one time point or short periods rather than in vivo or time lapse manner. In addition, this imaging system is frequently limited to two-dimensional imaging or very thin three-dimensional imaging (3). Two-photon confocal microscopy can be used to compensate the limitations of conventional single-photon confocal microscopy and enables researchers to investigate deeper regions of the tissues for as long as several hours, particularly in live animals (7). Confocal microscopy typically suggests the use of single-photon confocal microscopy; “two-photon microscopy” is widely used to describe two-photon confocal microscopy.
Since two-photon microscopic imaging shows a lower probability of causing phototoxicity, it is considered an essential tool in intravital imaging for long periods from several hours through a few days (8). In addition, researchers can analyze actual morphology and motility of live specimens in the deep area of the tissues because of the ability of two-photon microscopy to penetrate deep inside the tissue up to 1 millimeter. The capability to obtain spatiotemporal information such as the motility and morphology of target cells and tissues is only possible using intravital imaging, which reveals the dynamics of living targets of intact organisms (7). Leukocyte activity is one of the most dynamic phenomena in live animals. Therefore, many researchers use the features of two-photon microscope to determine the mechanism and pathology of leukocytes in the immune response.
When tissue is physically damaged or foreign substances such as bacteria and virus invade the body of live specimens, the immune system is activated in order to reduce the damage and maintain the homeostasis in the body. The first stage of this system is innate immunity, which is non-specific to pathogens and responds rapidly. Several leukocyte populations are key players in this innate response. Among the various leukocytes, neutrophil identification at the site of infection is considered a hallmark of inflammation (9), as it is the first to arrive to the affected location (10). In the bone marrow of humans, nearly 1011 neutrophils are generated and emitted into the blood circulation each day (10). Neutrophils must penetrate the blood vessels in order to migrate damage to tissue under inflammatory conditions. The neutrophil migration cascade is composed of intravascular migration, extravasation, and interstitial migration.
The blood vessel wall is composed of the endothelial cell layer, endothelial basement membrane, and pericytes (Fig. 1). Therefore, neutrophils must enter between these arrangements, which may result in morphological modification. Pericytes are located at the abluminal side of microvessels (11). These cells are known to be involved in controlling capillary permeability (12). The endothelial basement membrane is composed of laminins and collagen IV, which serve as ligands for adhesion molecules on migrating leukocytes (1314). Thus, endothelial cells also interconnect macromolecules, supporting structures, and signaling properties of the vessel (10).
Intravascular migration refers to all processes inside blood vessels, including rolling, adhesion, crawling, and firm adhesion of leukocytes to the endothelial cell layer (Fig. 1).
A complex cascade of intravascular migration is initiated by selectin-mediated “tethering and rolling” of leukocytes to the luminal side of the endothelium (10). Leukocytes use L-selectin to interact with the endothelium and E-selectin attracts leukocytes to the endothelial cell layer near the injured area (15). Next, chemokines activate integrins such as VLA-4 and LFA-1 through G-protein coupled receptors on leukocytes (16). VLA-4 regulates leukocyte rolling and arrest, whereas LFA-1 enables firm adhesion to the endothelial surface (16). In addition, firm adhesion of neutrophils to the endothelium ligates endothelial cell adhesion molecules and their ligands including ICAM-1, VCAM-1, E-selectin, and P-selectin (17). These adhesion molecules are spatiotemporally regulated during each step of migration.
Following intravascular migration of leukocytes, neutrophils need to penetrate blood vessel wall. In this transendothelial migration process, multiple interactions are involved (10). After firm adhesion, neutrophils can cross the barrier of the blood vessel, which is composed of an endothelial cell layer and endothelial basement membrane with pericytes. Extravasation is composed of several sub-steps: intrusion, perivascular embedment and crawling, protrusion, uropod elongation, and finally tail detachment along with microparticle formation from the endothelial basement membrane (Fig. 1). Detailed sub-steps of extravasation will be discussed below. Extravasation is the actual step to overcome the vessel wall during neutrophil migration cascade. It is known that neutrophils undergo transendothelial migration in two different ways. One is the paracellular route which passes between the endothelial cell junctions while the other is the transcellular route which travels directly through the endothelial cell body (10). It was reported that majority of neutrophil transendothelial migration occurs via the paracellular route (17).
First, neutrophils that complete the crawling process intrude under the endothelial cell layer. Next, some neutrophils are embedded in the basement membrane area, while others migrate within the endothelial basement membrane, known as perivascular crawling. Finally, most neutrophils overcome the endothelial basement membrane through a specific location at which a small gap is formed between pericytes; this phenomenon is known as perivascular crawling and extension of ventral membrane protrusions. Neutrophils typically protrude through physically permissive sites rather than altering basement membrane formation. As neutrophils project throughout a small space, a uropod is formed and elongated. Recently, we found that the small end-tip of the uropod remains tied along the endothelial basement membrane region during tail retraction, forming microparticles (13). During this process, integrins interact to push and pull out the uropod. Particularly, LFA-1 mediates elongation and VLA-3 leads penetration through the basement membrane (13).
As neutrophil uropod elongation ends in accordance with microparticle formation beneath the basement membrane of the vessel, neutrophils can approach the desired area to prevent further damage to the tissue (Fig. 2).
Following the discovery of microparticle formation, we evaluated its role in vascular permeability because extravasation of neutrophils is closely related to diseases such as sepsis and organ failure. Well-controlled neutrophil and endothelial layer interactions do not cause serious problems in living organisms; however, when the system fails owing to severe inflammation, it can be fatal because of leakage of the plasma and other substances (1819). By measuring fluorescent dextran leakage from blood vessels in the presence of an LFA-1 blocking antibody outside the blood vessel with two-photon intravital imaging, we showed that LFA-1-dependent microparticle formation during neutrophil extravasation has crucial role in controlling vascular permeability. From this intravital study of vascular barrier function, we found that neutrophil-derived microparticles on the subendothelium may play a role as protective ‘seals’ for blood vessels (20). However, the characteristics of microparticles should be further investigated. Apart from the vascular barrier function of extravasating neutrophil-derived microparticles, we recently reported that neutrophil-derived microparticles lead to CD8+ T cell migration (21222324). It is widely known that the innate immune system is activated first, followed by the adaptive immune system when viral infection occurs (2526). Therefore, our results provide insight into the linkage between innate and adaptive immune responses in viral infection.
Two-photon microscopy has opened a new era in leukocyte migration research by enabling time-lapse deep tissue intravital imaging. The morphology and motility of leukocytes can be visualized to capture the real-time activity of cells in the intact tissue of live animals. Actually using this machinery, novel features of leukocyte subsets such as neutrophils, T cells, macrophages, and dendritic cells in the immune response have been recently discovered. Therefore, intravital imaging under two-photon microscopy will reveal more details of the immune response by visualizing the phenotypical morphology and functional relevance of immune cells.
Figures and Tables
 | Figure 1Schema of neutrophil extravasation cascade. In the process of intravascular migration, neutrophil tethers itself to the endothelial cells via (1) rolling, (2) adhesion and (3) crawling. Thereafter neutrophil is (4) firmly adhered to the luminal surface of the vessel. After approaching to the proper site of extravasation, (5) leukocyte transmigrate through the endothelial cells, pericyte and basement membrane. In this process of extravasation, neutrophil undergoes (a) intrusion, (b) perivascular embedment & crawling, (c) protrusion, and then finally (d) uropod elongation & microparticle formation. Microparticles (red dot) are formed in this stage, and usually embedded between endothelial cells and pericytes. When extravasation is over, (6) leukocyte starts interstitial migration. |
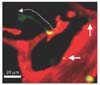 | Figure 2Uropod elongation and microparticle formation during neutrophil extravasation. The cremaster muscle of WT mouse was stimulated by CXCL2 post to intravenous injection of Alexa Fluor 488-anti-Gr1 antibody and Texas Red-dextran. The representative image shows uropod elongation of an extravasating neutrophil (dotted line with arrow) and deposited microparticles at the subendothelium (arrows) from neutrophils. |
ACKNOWLEDGEMENTS
This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2016 (2016-32-0031), a faculty research grant of Yonsei University College of Medicine (6-2016-0132), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2016R1A2B4008199) (Y-M. H.).
References
1. Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002; 2:872–880.

4. Stephens DJ, Allan VJ. Light microscopy techniques for live cell imaging. Science. 2003; 300:82–86.

5. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011; 12:761–769.

6. McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010; 330:362–366.

7. Ishii T, Ishii M. Intravital two-photon imaging: a versatile tool for dissecting the immune system. Ann Rheum Dis. 2011; 70:Suppl 1. i113–i115.

8. Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014; 505:223–228.

9. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006; 203:2569–2575.

10. Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013; 5:336–347.

11. Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998; 53:637–644.

12. Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012; 45:327–347.

13. Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012; 209:1349–1362.

14. Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, Sonnenberg A, Sarangi PP, Kim M. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin alpha3beta1-dependent. Blood. 2014; 24:3515–3523.
16. Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009; 122:215–225.

17. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009; 30:547–556.

18. He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res. 2010; 87:281–290.

19. Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981; 289:646–650.

20. Lim K, Sumagin R, Hyun YM. Extravasating neutrophil-derived microparticles preserve vascular barrier function in inflamed tissue. Immune Netw. 2013; 13:102–106.

21. Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002; 99:2703–2711.

22. Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007; 204:605–618.

23. Pi X, Wu Y, Ferguson JE III, Portbury AL, Patterson C. SDF-1alpha stimulates JNK3 activity via eNOS-dependent nitrosylation of MKP7 to enhance endothelial migration. Proc Natl Acad Sci U S A. 2009; 106:5675–5680.

24. Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, Kim M. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015; 349:aaa4352.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download