Abstract
It has been reported that fatty acid binding proteins (FABPs) do not act only as intracellular mediators of lipid responses but also have extracellular functions. This study aimed to investigate whether extracellular liver type (L)-FABP has a biological activity and to determined serum L-FABP levels in patients with end-stage renal disease (ESRD). We isolated L-FABP complementary deoxyribonucleic acid (cDNA) from the Huh7 human hepatocarcinoma cell line and expressed the recombinant L-FABP protein in Escherichia coli. A549 lung carcinoma and THP-1 monocytic cells were stimulated with the human recombinant L-FABP. Human whole blood cells were also treated with the human recombinant L-FABP or interleukin (IL)-1α. IL-6 levels were measured in cell culture supernatants using IL-6 enzyme-linked immunosorbent assay (ELISA). Human recombinant L-FABP induced IL-6 in a dose-dependent manner in A549, THP-1 cells, and whole blood cells. The blood samples of healthy volunteers and patients with ESRD were taken after an overnight fast. The serum levels of L-FABP in healthy volunteers and ESRD patients were quantified with L-FABP ELISA. The values of L-FABP in patients with ESRD were significantly lower than those in the control group. Our results demonstrated the biological activity of L-FABP in human cells suggesting L-FABP can be a mediator of inflammation.
Fatty acid-binding proteins (FABPs) are a family of cytosolic proteins with a molecular weight of 14 to 15 kDa that are involved in the intracellular lipid responses in cells (1). To date nine different FABPs have been identified and named according to the tissues in which they were first identified, for example, liver-type (L-), intestinal-type (I-), heart-type (H-), adipocyte-type (A-), epidermal-type (E-), and brain-type (B-) FABP (1). Among these, the first described FABP, liver-type FABP (L-FABP) is highly expressed in the liver (2~5% of cytosolic protein) as well as in the kidney, lung, pancreas and intestine (1), and a key regulator of hepatic lipid metabolism by influencing the uptake, transport, mitochondrial oxidation, and esterification of fatty acids (2).
Since FABPs lack a secretory signal sequence, these proteins are not detected in the blood stream under normal circumstances (34). Thus, some FABPs have been to be used as diagnostic markers of tissue injury: L-FABP for liver damage (567), I-FABP for intestinal injury (89), H-FABP for acute myocardial infarction and ongoing myocardial damage in heart failure (1011), and B-FABP for brain injury (12). Recent studies, however, have shown that some of FABPs can be secreted from cells without cell damage. L-FABP is detectable in bile in the absence of cellular injury (), H-, A-, and E-FABPs are present in milk (1415), and several recent reports have shown that A-FABP is secreted from adipocytes into the circulation or extracellular spaces (1617). Furthermore, it has become evident that the A-FABP after release from adipose tissue has crucial hormonal functions on liver, pancreas and heart (181920). As for the role of L-FABP, different studies have shown that urinary L-FABP in humans would be a useful clinical marker for acute kidney injury, and prediction and monitoring of the progression of chronic kidney disease (212223). Other reports suggested that serum L-FABP level may serve as an early biomarker for lung damage in a model of acute respiratory failure (24) and renal injury following kidney transplantation (25), and as a new diagnostic marker for detecting non-alcoholic fatty liver disease (26). In addition, several correlative studies suggest that L-FABP may also, like the A-FABP, function as an endocrine factor by showing that increased serum concentrations of L-FABP are associated with obesity, insulin resistance and high blood pressure (2728). However, little is known about the extracellular biological function of secreted L-FABP.
In the present study, we investigate whether L-FABP has a biological function in the blood and other human cells. In addition, we determined serum L-FABP levels in patients with end-stage renal disease (ESRD) on hemodialysis (HD), who have been known to have altered lipid metabolism including fatty acid.
A549 lung carcinoma and THP-1 monocytic cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). A549 cells were maintained according to the instructions and THP-1 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS). Huh7 cells were maintained in DMEM with 10% FBS. Human IL-6 enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems (Minneapolis, MI, USA).
Total ribonucleic acid (RNA) was isolated with TRI Reagent® (Sigma-Aldrich, St. Louis, MO, USA) from the Huh7, HepG2, HT29, colo205, sw620, KM12C cells (not shown). A pair of human L-FABP sense primers, 5'-ctacagtggacagtctggtc-3', and reverse primer, 5'-gtttaaa ttctcttgctgattc-3', was used for the RT-PCR. Moloney murine leukemia virus-reverse transcriptase (Beams Bio, Korea) was used for converting 2 µg of total RNA to first strand complementary deoxyribonucleic acid (cDNA), and the PCR was performed at 94℃ for 45 s, 70℃ for 2 min, and 56℃ for 1 min for 30 cycles.
For E. coli expression vector, the PCR product of human L-FABP cDNA was ligated into T&A cloning vector (RBC, Taiwan). The cDNA of L-FABP was amplified by PCR with the sense primers of human FABP1 with 4 overhangs, NdeI site with artificial start codon for cloning into pET21a, 5'-ATATCATATGAGTTTCTCCGGCAA- 3', and the reverse primer of human FABP1 with 4 overhangs and XhoI site for cloning into pET21a, 5'-AT ATCTCGAGaattctcttgctgatTCT-3'. Inserts were digested with NdeI and XhoI and then transferred into pET-21a (Invitrogen, Carlsbad, CA, USA) for recombinant protein expression in E. coli.
Human recombinant L-FABP protein was expressed in E. coli and Rosetta cells (Novagen, Madison, WI, USA) and purified by TALON affinity columns (Invitrogen) by using His6-tag at the N terminus of recombinant proteins. The TALON affinity-purified proteins were subjected to high performance liquid chromatography (HPLC, GE Healthcare) with a C4 column (Grace Vydac, Hesperia, CA, USA). The recombinant protein was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for silver staining. In addition, the eluents of various fractions and known amounts of bovine serum albumin (BSA) were subjected to 10% SDS-PAGE and visualized by silver staining. A standard curve was created with density measurement of BSA standards and used to calculate estimates of human recombinant L-FABP protein concentration.
ESRD patients had received HD in Jeju National University Hospital Dialysis Unit were studied age of 61.6±1.8 (mean±SEM) years. This study was approved by the institutional review board of Jeju National University Hospital and informed consent was obtained for the patients. The control group included age and gender matched healthy volunteers (n=63), renal diseases of the ESRD dialysis (n=75), and diabetes patients (n=87). The ESRD dialysis patients were dialyzed 2 or 3 times per week on Monday–Wednesday–Friday, Tuesday–ThursdaySaturday, or Tuesday–Saturday using high-flux membranes (dialysis filter surface area, 1.7~2.1 m2), and exhibited an equilibrated Kt/V of 2.07±0.23. The mean duration of HD was 63.4±7.1 months.
Blood samples of healthy volunteers were taken after an overnight fast and collected in heparin- or ethylenediamine tetraacetic acid (EDTA)-coated tube. And then we compared the effect of the two commonly used anticoagulants on the ability of human whole blood cells to induce IL-6 upon human recombinant L-FABP or IL-1α stimulation. Blood samples of ESRD patients were drawn before the start of a routine HD treatment on Monday or Tuesday and used to measure the levels of serum L-FABP. A549 (2.5×105/well), THP-1 (1×106/well) and human whole blood cells (1:2 dilution with RPMI) were seeded in a 96-well plate with 200 µl volume and then were treated with various concentrations of human recombinant L-FABP for overnight.
IL-6 levels were measured in cell culture supernatants by sandwich ELISA. For the detection of human L-FABP in serum, we developed monoclonal antibody against L-FABP from BALB/c mice immunized with the purified human recombinant L-FABP (not shown). The secondary antibody for L-FABP was conjugated to horseradish peroxidase with the use of succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate and a two-step sandwich ELISA method was carried out for determining L-FABP concentrations in serum. The detection limit was approximately 80 pg/ml.
In order to purify human recombinant L-FABP protein, the E. coli extract was applied to a mini Talon affinity column. The primarily purified recombinant L-FABP protein was further purified by HPLC. The molecular size of His6-tag recombinant L-FABP was approximately 16 kDa. HPLC results indicated two groups of peak after 51 and 63 min (Fig. 1). Aliquots of the various fractions were resolved by 10% SDS-PAGE, and the protein bands were visualized by silver staining. A serious of protein bands with molecular weight of 16-, 32-, and 48 kDa were identified in nearly all fractions examined (Fig. 2A). Especially these protein bands were detected mainly in fractions 63 and 64 (Fig. 2B). We verified human recombinant L-FABP in the eluted fractions with a monoclonal antibody specific to human L-FABP. The anti-human L-FABP monoclonal antibody recognized the 16-, 32-, and 48-kDa human L-FABP (not shown). The results suggest that 16,32 and 48 kDa are the monomer, dimer and trimer forms of L-FABP, respectively (not shown).
In addition, the eluents from fraction 47 to 54 (12 µl) and from fraction 63 to 64 (1 and 2 µl, respectively), and known amounts of BSA were subjected to 10% SDS-PAGE and visualized by silver staining in order to estimate human recombinant L-FABP concentration (Fig. 3). A standard curve was created with density measurement of BSA standards and used to calculate estimates of human recombinant L-FABP protein concentration (not shown). Purified human recombinant L-FABP proteins from fraction 47 to 54 typically migrated as monomer, dimer and trimer, otherwise those from fraction 63 and 64 mainly migrated as monomer and dimer (Fig. 3).
A549 and THP-1 cells were stimulated with various concentrations of human recombinant L-FABP for 24 h and then the induction of IL-6 was assessed by ELISA. The production of IL-6 was augmented along with the increased concentrations of human recombinant L-FABP in these cells, respectively (Fig. 4A and B). IL-6 production was also examined with human recombinant L-FABP or IL-1α treated human whole blood cells to investigate the biological activity of human recombinant L-FABP on human whole blood cells. As shown in Fig. 4C, human recombinant L-FABP or IL-1α stimulation increased IL-6 production. In addition, the effects of both human recombinant L-FABP and IL-1α were stronger in the human whole blood cells collected with heparin-coated tube than in those collected with EDTA-coated tube.
We determined serum L-FABP levels using our ELISA assay in healthy control group and compared them with those in ESRD patients on HD because these patients have been known to have altered lipid metabolism including fatty acid (2930) and so might have altered serum levels of L-FABP. The serum levels of L-FABP were decreased in the ESRD patients on HD compared with those in the control group (2.7 ng/ml vs. 0.23 ng/ml, p<0.001) in Fig. 5. Also the level of diabetes patients L-FABP was lower than the level of normal control group. Especially, most of protein levels of ESRD patients on HD were lower than the lower limit of the control group (not shown).
FABPs were originally described as intracellular proteins that can affect lipid fluxes, metabolism and signaling within cells (1). However, numerous studies have shown the presence of FABPs in circulation (272831). In general, these circulating FABPs have been considered as markers of cell injury or death because they have no secretory signal peptide. However, a growing body of evidence suggests that the circulating form of FABPs have crucial hormonal functions in other tissues without cell damage. Several correlation studies have reported that elevated serum levels of A-FABP were associated with obesity, type 2 diabetes, hypertension, and cardiovascular diseases (2832), suggesting that there can be the link between circulating levels of A-FABP, and metabolic and cardiovascular disease. And a previous study reported that A-FABP directly depresses cardiomyocyte contractile activity after release from adipose tissue (18). Furthermore, another study revealed that exogenous A-FABP can induce replication and migration of human coronary artery smooth muscle cells (33). Moreover, some recent studies reported that exogenous A-FABP to primary hepatocytes can induce gluconeogenesis (19), and insulin resistance without altering beta-cell responsiveness in the pancreas (20). Altogether, previous data suggest that A-FABP has some hormonal functions on other tissues after release from adipose tissue. As for other FABPs, a few studies suggest that some FABPs such as L-FABP and E-FABP may have roles in systemic metabolism and cardiovascular system by showing the association between their serum concentrations, and metabolic and cardiovascular regulation markers (27283435). However, it has not been investigated, to our knowledge, that whether the secreted form of L-FABP is biologically active. In order to investigate its biologic activity, if any, we stimulated A549 lung carcinoma, THP-1 monocytic and human whole blood cells with human recombinant L-FABP and measured IL-6 with ELISA, and showed that IL-6 was induced in a dose dependent manner in these cells. Especially human recombinant L-FABP induced IL-6 in whole blood cells similar with IL-1α known as representative inflammatory cytokine. These results suggest that L-FABP in circulation or extracelluar space may have a role as a mediator of inflammatory process although we could not find out the mechanism by which L-FABP enters the blood compartment and if cell surface molecules that interact with L-FABPs on blood cells exist.
Additionally we found that the effects of both human recombinant L-FABP and IL-1α were potent inducer of IL-6 in the human whole blood cells collected with heparin-coated tube than in those collected with EDTA-coated tube. EDTA has an anticoagulant effect in blood by chelating calcium which is necessary for a wide range of enzyme reactions of the coagulation cascade and has been frequently used as the anticoagulant of choice for investigating hematological parameters, as cell morphology is preserved best over time (36). However, the use of heparin is recommended as the anticoagulant of choice in whole blood cell experiments because its influence on immune cell functions has been lesser than other anticoagulants, and anticoagulants acting by the chelation of calcium ion such as EDTA can remove the role of calcium in the activation of immune cells (373839). Thus, our results support that removal of calcium ion by EDTA can result in lower cytokine expression capacity in the whole blood compared with the use of heparin.
It has been shown that elevated serum levels of several FABPs are associated with lower glomerular filtration rate in general population and patients with angina pectoris, suggesting that FABPs are eliminated from the circulation mainly by renal clearance (2840). In addition, some studies reported that serum concentrations of H-FABP or A-FABP are increased in ESRD patients on HD compared to those in control subjects with normal renal function (41424344). However, there are only 2 published studies that investigated L-FABP levels in chronic kidney disease (CKD). One study reported that serum L-FABP levels in patients with CKD were higher than those in healthy volunteers (5.4±4.4 ng/mL vs. 1.2±0.7 ng/mL), and correlated with serum creatinine, suggesting that it may accumulate in the circulatory system through a decrease in glomerular filtration rates (21). However, the renal function of CKD patients in their study was relatively mild (serum creatinine, 1.1±0.8 mg/dl), so it may not represent the serum concentrations of L-FABP in ESRD patients. The other recent study showed that serum L-FABP levels of ESRD patients on HD (n=67) were higher compared with those of historical healthy control group (17.9±3.1 ng/ml vs. 1.2±0.7 ng/ml) and that serum L-FABP can serve as a biomarker for renal injury after kidney transplantation and to predict graft recovery and the need for HD (25). However, they did not compare the concentrations of L-FABP of ESRD patients with those of their own healthy control group. Contrary to the results of previous two studies, serum levels of L-FABP in ESRD patients on HD were significantly lower than those in the healthy control group in our study. So far there is no study to investigate the underlying mechanisms of increase or decrease in serum L-FABP levels in ESRD patients. Thus, the effect of ESRD on serum levels of L-FABP in patients remains unresolved. However, some studies reported that liver in rats with advanced CKD induced by 5/6 nephrectomy exhibits reduced nuclear peroxisome proliferator-activated receptor-α (PPARα) and downregulation of L-FABP which is upregulated by PPARα ligands (3045). Moreover fibrates stimulate cellular fatty acid uptake and catabolism by the beta-oxidation pathways through PPARα activation which are main roles of L-FABP in the liver (46). Based on these reports, we speculated that reduced activities of PPARα and L-FABP genes induced by ESRD may cause the reduced serum levels of L-FABP in ESRD patients.
In conclusion, we showed the biological activity of human L-FABP by demonstrating that its human recombinant form induces IL-6 production in human cell lines and whole blood cells. And the similar effect of L-FABP with IL-1α on whole blood cells indicates that circulating or extracellular form of L-FABP, if any, can be a mediator of systemic inflammation. In addition, we also found that the serum levels of L-FABP in ESRD patients on HD were significantly lower than those in the healthy control group, suggesting that severe renal dysfunction may affect the decreased induction of L-FABP in the liver.
Figures and Tables
 | Figure 1Isolation of human recombinant liver-fatty acid binding protein by using high performance liquid chromatography (HPLC). Human recombinant L-FABP was expressed in E. coli and first purified by a Talon metal affinity chromatography (not shown). Then the protein was further purified by HPLC. The peaks corresponding to human recombinant L-FABP were observed right after 51 and 63 min. Units are in milli Absorbance Units (mAU) at 280 nm. The data presents one of five independent experiments. |
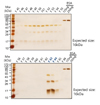 | Figure 2Silver staining of human recombinant liver-fatty acid binding protein. The purified human recombinant liver-fatty acid binding protein was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and visualized by silver staining. (A) The eluants from fraction 45 to 55 were visualized by silver stain. A serious of protein bands with molecular weight of 16- (monomer form), 32- (dimer form), and 48 kDa (trimer form) were identified in nearly all fractions examined. (B) These protein bands were detected mainly in fractions 63 and 64 especially. The data represent one of three independent experiments. Molecular weight (Mw) is indicated on the left. BSA; bovine serum albumin, kDa; kilodalton. |
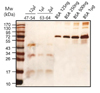 | Figure 3Quantification of human recombinant liver-fatty acid binding protein concentration. The purified human recombinant L-FABP was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, visualized by silver staining, and compared with various known amount of bovine serum albumin (BSA). Twelve µl of the eluants from fraction 47 to 54 was loaded in the second lane. One and 2 µl of the eluants from fraction 63 to 64 was loaded in the third and fourth lanes, respectively. Purified human recombinant L-FABP proteins from fraction 47 to 54 typically migrate as monomer, dimer, and trimer otherwise those from fraction 63 and 64 mainly migrate as monomer and dimer. A standard curve was created with density measurement of BSA standards and used to calculate estimates of human recombinant L-FABP protein concentration. The data represent one of three independent experiments. Molecular weight (Mw) is indicated on the left. BSA; bovine serum albumin, kDa; kilodalton. |
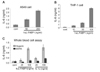 | Figure 4Biological activities of human recombinant liver-fatty acid binding protein. Human recombinant L-FABP was examined with A549 lung carcinoma and THP-1 monocytic and human whole blood cells. A549 cells (A) and THP-1 cells (B) were treated with various concentrations of human recombinant L-FABP as indicated under the horizontal axis for 24 h, and interleukin IL-6 levels were assessed by enzyme-linked immunosorbent assay (ELISA). Human recombinant L-FABP induced IL-6 in a dose-dependent manner in these cells. (C) Whole bloods from healthy volunteers were collected with heparin- or ethylenediamine tetraacetic acid (EDTA)-coated tube and IL-6 supernatant levels were measured with ELISA after 24 h of incubation with human recombinant L-FABP or IL-1α at concentrations indicated under the horizontal axis. Both the human recombinant L-FABP and IL-1α induced IL-6 in human whole blood cells compared with non-treated control cells. These effects were stronger in the blood cells collected with heparin-coated tube than in those collected with EDTA-coated tube. The data represent one of three independent experiments. cont, control; hr, human recombinant; L-FABP, liver-fatty acid binding protein. |
 | Figure 5Liver-fatty acid binding protein concentration in human serum. The serum L-FABP levels of the healthy control group (n=63), renal diseases of the ESRD dialysis (n=75), and diabetes patients (n=87). Blood samples of healthy volunteers were taken after an overnight fast and those of ESRD patients were drawn before the start of a routine hemodialysis treatment. Levels were measured using our ELISA assay. The values of L-FABP in patients with ESRD were significantly lower than those in the control group (2.7 ng/ml vs. 0.23 ng/ml, p<0.001). ESRD, end-stage renal disease; L-FABP, liver-fatty acid binding protein. |
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF- 2015R1A2A2A01003472, -2014M3A6A4075058, -2015R1A2A1A15051472) and this paper resulted from the Konkuk University research support program. This work was done while the first author's his research year of Jeju National University in 2015. This paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2014.
References
1. Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008; 7:489–503.

2. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem. 2010; 21:1015–1032.

3. Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996; 110:339–343.

4. Piumngam P, Schachtrup C, Owada Y, Kondo H, Promptmas C, Spener F. Expression of liver-type fatty acid-binding protein in murine lung and its release into serum upon challenge of lung with lipopolysaccharide. Eur J Lipid Sci Technol. 2005; 107:145–152.

5. Pelsers MM, Morovat A, Alexander GJ, Hermens WT, Trull AK, Glatz JF. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin Chem. 2002; 48:2055–2057.

6. Monbaliu D, de VB, Crabbe T, van HE, Verwaest C, Roskams T, Fevery J, Pirenne J, Buurman WA. Liver fatty acid-binding protein: an early and sensitive plasma marker of hepatocellular damage and a reliable predictor of graft viability after liver transplantation from non-heart-beating donors. Transplant Proc. 2005; 37:413–416.

7. Akbal E, Koklu S, Kocak E, Cakal B, Gunes F, Basar O, Tuna Y, Senes M. Liver fatty acid-binding protein is a diagnostic marker to detect liver injury due to chronic hepatitis C infection. Arch Med Res. 2013; 44:34–38.

8. Morrissey PE, Gollin G, Marks WH. Small bowel allograft rejection detected by serum intestinal fatty acid-binding protein is reversible. Transplantation. 1996; 61:1451–1455.

9. Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JF. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003; 36:529–535.

10. Okamoto F, Sohmiya K, Ohkaru Y, Kawamura K, Asayama K, Kimura H, Nishimura S, Ishii H, Sunahara N, Tanaka T. Human heart-type cytoplasmic fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. Clinical evaluation of H-FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin Chem Lab Med. 2000; 38:231–238.

11. Setsuta K, Seino Y, Ogawa T, Arao M, Miyatake Y, Takano T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am J Med. 2002; 113:717–722.

12. Pelsers MM, Hanhoff T, d Van V, Arts B, Peters M, Ponds R, Honig A, Rudzinski W, Spener F, de K Jr, Twijnstra A, Hermens WT, Menheere PP, Glatz JF. Brain- and heart-type fatty acid-binding proteins in the brain: tissue distribution and clinical utility. Clin Chem. 2004; 50:1568–1575.

13. Foucaud L, Grillasca J, Niot I, Domingo N, Lafont H, Planells R, Besnard P. Output of liver fatty acidbinding protein (L-FABP) in bile. Biochim Biophys Acta. 1999; 1436:593–599.

14. Specht B, Bartetzko N, Hohoff C, Kuhl H, Franke R, Borchers T, Spener F. Mammary derived growth inhibitor is not a distinct protein but a mix of heart-type and adipocyte-type fatty acid-binding protein. J Biol Chem. 1996; 271:19943–19949.

15. Bronsky J, Karpisek M, Bronska E, Pechova M, Jancikova B, Kotolova H, Stejskal D, Prusa R, Nevoral J. Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: proteins newly identified in human breast milk. Clin Chem. 2006; 52:1763–1770.

16. Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006; 52:405–413.

17. Mita T, Furuhashi M, Hiramitsu S, Ishii J, Hoshina K, Ishimura S, Fuseya T, Watanabe Y, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring). 2015; 23:359–367.

18. Lamounier-Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, Ehrhart-Bornstein M, Bornstein SR, Morano I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009; 105:326–334.

19. Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, Tuncman G, Hotamisligil GS. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013; 17:768–778.

20. Kralisch S, Kloting N, Ebert T, Kern M, Hoffmann A, Krause K, Jessnitzer B, Lossner U, Sommerer I, Stumvoll M, Fasshauer M. Circulating adipocyte fatty acid-binding protein induces insulin resistance in mice in vivo. Obesity (Silver Spring). 2015; 23:1007–1013.

21. Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Omata M, Kimura K. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem. 2006; 284:175–182.

22. Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007; 18:2894–2902.

23. Matsui K, Kamijo-Ikemori A, Imai N, Sugaya T, Yasuda T, Tatsunami S, Toyama T, Shimizu M, Furuichi K, Wada T, Shibagaki Y, Kimura K. Clinical significance of urinary liver-type fatty acid-binding protein as a predictor of ESRD and CVD in patients with CKD. Clin Exp Nephrol. 2016; 20:195–203.

24. Lachmann RA, Werchan S, Schachtrup C, Haitsma JJ, Spener F, Lachmann B. Liver-type fatty acid binding protein in serum and broncho-alveolar lavage in a model of acute respiratory failure because of surfactant depletion--a possible marker for lung damage? Clin Physiol Funct Imaging. 2006; 26:371–375.

25. Kawai A, Kusaka M, Kitagawa F, Ishii J, Fukami N, Maruyama T, Sasaki H, Shiroki R, Kurahashi H, Hoshinaga K. Serum liver-type fatty acid-binding protein predicts recovery of graft function after kidney transplantation from donors after cardiac death. Clin Transplant. 2014; 28:749–754.

26. Akbal E, Kocak E, Akyurek O, Koklu S, Batgi H, Senes M. Liver fatty acid-binding protein as a diagnostic marker for non-alcoholic fatty liver disease. Wien Klin Wochenschr. 2016; 128:48–52.

27. Shi J, Zhang Y, Gu W, Cui B, Xu M, Yan Q, Wang W, Ning G, Hong J. Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PLoS One. 2012; 7:e48777.

28. Ishimura S, Furuhashi M, Watanabe Y, Hoshina K, Fuseya T, Mita T, Okazaki Y, Koyama M, Tanaka M, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Miura T. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One. 2013; 8:e81318.

29. Kwan BC, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007; 18:1246–1261.

30. Jin K, Norris K, Vaziri ND. Dysregulation of hepatic fatty acid metabolism in chronic kidney disease. Nephrol Dial Transplant. 2013; 28:313–320.

31. Fuseya T, Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Mita T, Ishimura S, Watanabe Y, Hoshina K, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol. 2014; 13:126.

32. Yeung DC, Xu A, Tso AW, Chow WS, Wat NM, Fong CH, Tam S, Sham PC, Lam KS. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care. 2009; 32:132–134.

33. Girona J, Rosales R, Plana N, Saavedra P, Masana L. FABP4 induces vascular smooth muscle cell proliferation and migration through a MAPK-dependent pathway. PLoS One. 2013; 8:e81914.

34. Yeung DC, Wang Y, Xu A, Cheung SC, Wat NM, Fong DY, Fong CH, Chau MT, Sham PC, Lam KS. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J. 2008; 29:2156–2163.

35. Bagheri R, Qasim AN, Mehta NN, Terembula K, Kapoor S, Braunstein S, Schutta M, Iqbal N, Lehrke M, Reilly MP. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am J Cardiol. 2010; 106:1118–1123.

36. Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014; 24:31–44.

37. Brunialti MK, Kallas EG, Freudenberg M, Galanos C, Salomao R. Influence of EDTA and heparin on lipopolysaccharide binding and cell activation, evaluated at single-cell level in whole blood. Cytometry. 2002; 50:14–18.

38. Duvigneau JC, Sipos W, Hartl RT, Bayer M, Moldzio R, Stevenson L, Adair B, Gemeiner M. Heparin and EDTA as anticoagulant differentially affect cytokine mRNA level of cultured porcine blood cells. J Immunol Methods. 2007; 324:38–47.

39. Freitas M, Porto G, Lima JL, Fernandes E. Isolation and activation of human neutrophils in vitro The importance of the anticoagulant used during blood collection. Clin Biochem. 2008; 41:570–575.

40. Iwamoto M, Miyoshi T, Doi M, Takeda K, Kajiya M, Nosaka K, Nakayama R, Hirohata S, Usui S, Kusachi S, Sakane K, Nakamura K, Ito H. Elevated serum adipocyte fatty acid-binding protein concentrations are independently associated with renal dysfunction in patients with stable angina pectoris. Cardiovasc Diabetol. 2012; 11:26.

41. Furuhashi M, Ura N, Hasegawa K, Yoshida H, Tsuchihashi K, Nakata T, Shimamoto K. Serum ratio of heart-type fatty acid-binding protein to myoglobin A novel marker of cardiac damage and volume overload in hemodialysis patients. Nephron Clin Pract. 2003; 93:C69–C74.
42. Furuhashi M, Ura N, Hasegawa K, Tsuchihashi K, Nakata T, Shimamoto K. Utility of serum ratio of heart-type fatty acid-binding protein to myoglobin for cardiac damage regardless of renal dysfunction. Circ J. 2004; 68:656–659.

43. Sommer G, Ziegelmeier M, Bachmann A, Kralisch S, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of adipocyte fatty acid-binding protein (AFABP) are increased in chronic haemodialysis (CD). Clin Endocrinol (Oxf). 2008; 69:901–905.

44. Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, Yoshida H, Shimamoto K, Hotamisligil GS, Miura T. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 2011; 6:e27356.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download