Abstract
Thymic atrophy is a complication that results from exposure to many environmental stressors, disease treatments, and microbial challenges. Such acute stress-associated thymic loss can have a dramatic impact on the host's ability to replenish the necessary naïve T cell output to reconstitute the peripheral T cell numbers and repertoire to respond to new antigenic challenges. We have previously reported that treatment with the orexigenic hormone ghrelin results in an increase in the number and proliferation of thymocytes after dexamethasone challenge, suggesting a role for ghrelin in restraint stress-induced thymic involution and cell apoptosis and its potential use as a thymostimulatory agent. In an effort to understand how ghrelin suppresses thymic T cell apoptosis, we have examined the various signaling pathways induced by receptor-specific ghrelin stimulation using a restraint stress mouse model. In this model, stress-induced apoptosis in thymocytes was effectively blocked by ghrelin. Western blot analysis demonstrated that ghrelin prevents the cleavage of pro-apoptotic proteins such as Bim, Caspase-3, and PARP. In addition, ghrelin stimulation activates the Akt and Mitogen-activated protein kinases (MAPK) signaling pathways in a time/dose-dependent manner. Moreover, we also revealed the involvement of the FoxO3a pathway in the phosphorylation of Akt and ERK1/2. Together, these findings suggest that ghrelin inhibits apoptosis by modulating the stress-induced apoptotic signal pathway in the restraint-induced thymic apoptosis.
The thymus is an essential immune system organ required for generating T lymphocytes, a developmental process that is maintained throughout adolescence and early adulthood but appears to progressively decline with advancing age (12). The Development of thymocytes are extremely sensitive to stress such as infections, the environment, and immunosuppressive treatments, including cancer, radiation therapy, and/or surgery, which can result in the apoptosis of developing thymocytes (345). Thymus atrophy induced by different types of stress is characterized by a reduction in thymus size caused by a sharp drop in thymocyte number, and reduced output of naïve T cells to the periphery (6). However, mechanisms that drive thymic involution are poorly understood. Moreover, there are no treatments available to either protect against thymic atrophy or accelerate recovery, thereby leaving the immune system compromised during various stress events.
We focused on ghrelin, a 28-amino acid hunger-stimulating peptide hormone, which plays a major role in the secretion of growth hormones and regulation of food intake (78). Ghrelin has been demonstrated to mediate a number of diverse biological functions both in vivo and in vitro, which extend beyond its effects on the central nervous system (9101112). For example, ghrelin has been shown to increase cell proliferation and inhibit in vitro apoptosis of a number of cell types including cardiomyocytes, endothelial cells, and enterocytes and several tumor cells (71314). Ghrelin is now known to have a wide spectrum of effects on the endocrine, reproductive, and cardiovascular systems. It is expected that additional functions of this potent hormone will be identified given the wide, albeit quantitatively variable, distribution of GHS-Rs throughout a variety of tissues and cell types (1415). Recent studies have also demonstrated that ghrelin affects thymic biology (216).
In the current study, we found that ghrelin partially reverses restraint stress-induced thymic involution and apoptosis in vivo. Specific analysis of the signaling pathways associated with the anti-apoptotic effects of ghrelin revealed that GHSR-1a-specific ligation results in the activation of the AKT and FoxO3a signaling pathways, as well as in the suppression of the cleavage of Bim, Caspase-3, and PARP on restraint stress-induced murine thymocytes. Ghrelin-mediated activation of these pathways results in enhanced T cell survival and recovery from restraint stress-induced thymic atrophy.
Six-eight-week-old male C57BL/6 (B6) mice were purchased from Orient Bio Inc. (Gyeonggi, South Korea), and all animal experiments were carried out after receiving approval from the Korea University Institutional Animal Care and Use Committee. The mice were housed 5 per group, divided into each of the cages in the animal laboratory, under a 12-hour light/dark cycle, at 22±1℃.
We utilized a previously established restraint stress protocol with some modifications (17). Briefly, mice were injected intraperitoneally with 100 µg/kg of ghrelin or 10 mg/kg of mifepristone (Tocris Bioscience) in 0.5% carboxymethyl cellulose (CMC) (Sigma Aldrich) the day before the immobilization stress experiment (day–1). After 24 hr, mice were subjected to another ghrelin or mifepristone treatment 30 min before the start of the immobilization. Mice were then immobilized by placing them in disposable plastic restraints for 1.5 hr. This procedure was then repeated daily for 7 days. Animals were sacrificed by CO2 asphyxiation after completing the stress procedure. Thymus glands were removed from mice and were weighed.
Thymocytes were isolated from extracted thymus tissue using a cell strainer with the plunger of a syringe. Cells were centrifuged at 1,200 rpm for 5 min and the supernatant was removed. The cell pellet was then washed once in cold PBS and cells were resuspended in cold Flow Cytometry staining buffer (eBioscience, San Diego, CA, USA). Total thymocytes were counted using the Z1 COULTER COUNTER (Beckman Coulter, Inc., Brea, CA, USA).
Thymic lymphocyte apoptosis upon restraint stress was measured using the Annexin V-FITC Apoptosis Detection kit (BD Bioscience, San Jose, CA, USA) and analyzed by Flow Cytometry (BD Bioscience). After inducting restraint stress as described above, restraint stress-induced thymocytes were stained with Annexin V-FITC and propidium iodide (PI) at room temperature for 15 min. Stained cells were analyzed by a BD FACS Canto II cytometry system with FlowJo software (v7.6.5, Tree Star, Inc., Ashland, OR, USA).
Cells were lysed with lysis buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet p-40, 10% glycerol, 60 mM octyl β-glucoside, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM nitrophenylphosphate, 0.7 µg/ml pepstatin, and protease inhibitor cocktail tablet. For immunoprecipitation, lysates were mixed with an antibody against FoxO3a or 14-3-3 at 4℃ overnight, followed by the addition of 30 µl protein A or G-Sepharose beads for 3 hr at 4℃. After washing the immune complexes with PBS containing 0.05% Tween-20 (PBS-T) wash buffer at least three times, tubes were boiled in sample loading buffer and analyzed by western blotting. For western blotting, lysates were separated by SDS–PAGE and then transferred onto PVDF membranes. The membranes were blocked in 5% nonfat dry milk in TBS-T for 1 hr, and then incubated with the appropriate primary antibody at 4℃ overnight, followed by incubation with horseradish peroxidase–coupled secondary antibodies for 1 h. After washing, signals were detected using Luminata Crescendo Western HRP substrate (Merck Millipore, Darmstadt, Germany); then images were obtained using an ImageQuant LAS 4000 system (Fujifilm, Tokyo, Japan). The following antibodies were used: anti-Bim, anti-Bax, anti-BCL-2, anti-BCL-xL (Santa Cruz Biotechnology), anti-caspase-3, anti-PARP, anti-phospho-Akt, anti-phospho-FoxO3a, anti-FoxO3a, anti-phospho-Erk1/2, anti-phospho-p38, anti-phospho-MEK1/2 and anti-horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, anti-horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, and anti-horseradish peroxidase (HRP)-conjugated Actin (Cell Signaling Technology, Danvers, MA, USA).
Stress-induced thymic involution is characterized by reduction in thymus size caused by acute loss of thymocytes and thymus weight loss (6). As shown Fig. 1, ghrelin treatment suppressed the decrease in both thymus weight and cell number resulting from immobilization (Fig. 1A-C); in contrast, the PBS control group showed decreases in these parameters as expected. We next assessed the percentage of cortical thymocytes using flow cytometry. Ghrelin treatment significantly ameliorated the loss of thymocytes including double-positive cells (DP) during stress events (Fig. 1D, E).
To evaluate the inhibitory effect of ghrelin on restraint stress-induced thymocyte loss, we tested whether ghrelin treatment could affect apoptosis using annexin-V and PI staining analysis. As shown in Fig. 2, the percentage of apoptotic cells was significantly increased after restraint stress (white bar) compared with normal controls (black bar). However, when ghrelin was administered before restraint stress, the apoptotic rate was effectively reduced (p<0.01). These data suggest that ghrelin effectively protects thymocytes from apoptosis during restraint stress events.
To examine the molecular mechanism by which ghrelin suppresses apoptosis during stress-induced thymic involution, we next carried out western blotting on a panel of normal control and stress-induced thymocytes. As shown in Fig. 3A, ghrelin decreased the cleavage of Bim, Caspase-3, and PARP1 in restraint stress conditions, whereas expression of Bax was not affected by ghrelin. Furthermore, the expression of anti-apoptotic proteins, Bcl-2 and Bcl-xL, did not appear to change upon restraint stress. Thus, we expected that ghrelin is directly affecting apoptotic signaling pathways.
Forkhead box O3a (FoxO3a) is an important regulator of cell apoptosis that upregulates pro-apoptotic proteins such as Fas ligand and Bim (1819). Serine/threonine kinase Akt (PKB)-mediated phosphorylation of FoxO3a inhibits FoxO3a activity. This inhibitory phosphorylation of FoxO3a is associated with 14-3-3 sequestration, and this inhibition of FoxO3a transcription factors induces Bim expression (1920). As expected, ghrelin treatment resulted in phosphorylation of S473-Akt and S253-FoxO3a in restraint stress-induced apoptosis (Fig. 3B). These data indicate that ghrelin inhibits the activity of pro-apoptotic proteins by inhibiting the Akt/FoxO3a/Bim signaling pathway in restraint stress-induced thymocyte apoptosis.
To determine the direct relationship between ghrelin and Akt/FoxO3a/Bim-dependent anti-apoptotic effects, we next examined the effects of ghrelin on the phosphorylation of FoxO3a, Akt, and mitogen-activated protein kinases (MAPKs) by western blot analyses using phospho-specific antibodies. Thymocytes were prepared from mouse thymus tissues, and re-suspended in RPMI 1640 supplemented with 10% heat-inactivated FBS (Hyclone) and 50 µM β-ME. As revealed by western blotting, the phosphorylation of Akt and MAPKs increased upon ghrelin treatment (Fig. 4A), and the ghrelin-mediated phosphorylation of Akt and its downstream signaling molecules was significantly inhibited by Akt inhibitor IV (Fig. 4B). These results support our previous data that ghrelin inhibits FoxO3a activity via Akt-dependent phosphorylation of FoxO3a. The results in Fig. 4C reveal that ghrelin treatment significantly increased the level of FoxO3a phosphorylation at Ser253, Ser318/21, and Thr32.
Previous studies have demonstrated that the G-protein coupled growth hormone secretagogue receptor-1a (GHS-R1a) acts as binding partner for ghrelin (721). To evaluate whether this ghrelin receptor was highly involved in ghrelin-dependent Akt phosphorylation and its downstream signaling cascade to protect against apoptosis, we evaluated the effects of the selective GHS-R antagonist [D-Lys3]-Growth Hormone Releasing Peptide-6 (DLS). As shown in Fig. 4D, [D-Lys3]-GHRP-6 markedly inhibited the phosphorylation of Akt, MEK1/2, and Erk1/2 induced by ghrelin in thymocytes. This indicated that the effects of ghrelin on Akt/FoxO3a signaling are dependent on GHS-R1a.
As mention above, Akt activation induces binding of FoxO3a and 14-3-3, which can affect the function of proapoptotic protein-producing transcription factors such as Bim (20). We thus tested whether ghrelin mediates the interaction between FoxO3a and 14-3-3 using immunoprecipitation analysis. As shown in Fig. 5, ghrelin promoted the binding of FoxO3a to 14-3-3. These data showed that ghrelin offers a cell survival advantage by promoting binding of 14-3-3 to Akt-phosphorylated FoxO3a.
In the present study, we observed that ghrelin exerts an inhibitory effect on restraint stress-induced thymus atrophy in mice. The application of ghrelin effectively controlled the stress-induced loss of thymus weight and thymocyte number loss or damage (Fig. 1). These results indicated the physiological efficacy of ghrelin on the thymic involution employed in the present study.
In a previous study, we showed that ghrelin induced PI3K/Akt and Erk 1/2 phosphorylation, and these two signaling molecules play a role in lymphocyte activation and proliferation (7). Another group showed that activation of this pathway mediates ghrelin's inhibitory effect on endothelial cell apoptosis (22). Similar effects have been observed in cardiomyocytes, neuronal cell populations, 3T3-L1 cells, and several tumor cell types (232425). Collectively, these data suggest that ghrelin is important for thymus homeostasis. However, conflicting data have been reported recently regarding the anti-apoptotic effects of ghrelin in different cell types such as colon adenocarcinoma and human umbilical vein endothelial cells (2627).
Our present findings suggest that ghrelin reduced the apoptosis of thymic murine T cells upon in vivo (Fig. 2) and in vitro (Fig. 3) ghrelin administration, which is mediated by phosphorylation and inactivation of FoxO3a by Akt and Erk (Fig. 4). FoxO3a, an important transcription factor that contributes to FasL- and Bim-induced apoptosis (181920), is a downstream target of Akt and is negatively regulated by several upstream kinases including Akt and Erk (28). FoxO3a binds the adapter protein 14-3-3 upon Akt phosphorylation, which disrupts the trafficking of FoxO3a into the nucleus (29). This role of 14-3-3 protein is essential for cell survival and suppresses apoptosis from diverse stimuli. Interestingly, our results conclusively showed ghrelin-mediated inhibitory phosphorylation of FoxO3a and direct binding to 14-3-3 (Fig. 5).
Overall, our data provide a more comprehensive view of ghrelin's effects on acute stress-induced thymus atrophy. This protective effect suggested that ghrelin may be a beneficial therapeutic agent in clinical settings of immune reconstitution.
Figures and Tables
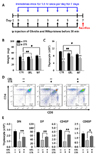 | Figure 1Experimental design and the result of behavioral assessments. (A) A diagrammatic representation of the experimental schedule. Mice were treated with ghrelin and mifepristone from D-1 to D+6 30 min before restraint stress. Mice were immobilized for 1.5 hr daily over a period of 7 days. (B, C) The weight of thymus (mg) and thymus cell numbers were measured at D+7 of restraint stress. (D, E) Percentage of thymic lymphocyte populations in the thymus after restraint stress. Flow cytometry plot (C) showing the gating strategy for thymocyte subsets. DN, double-negative; DP, double-positive; CD4SP, single-positive CD4; and CD8SP, single-positive CD8 thymocytes, respectively. Each bar represents the mean±SEM from 10 animals per group. *p<0.05, **p<0.01 for control (–STR) versus stressed (+STR) groups; #p<0.05, ##p<0.01 for stressed (+STR) groups. |
 | Figure 2The thymic protective effects of ghrelin upon restraint stress. (A, B) Flow cytometry scatter plots for thymic cells from one control (left) and one stressed (right) mouse. (A) Total cells, (B) CD4/CD8-double positive cells. |
 | Figure 3Effects of ghrelin treatment on the levels of apoptosis-related 14-3-3-sequestered proteins in restraint stress-induced thymic apoptosis. (A, B) Cell lysates were obtained from control (–STR) versus stressed (+STR) thymus tissues, and were subjected to western blot analysis with indicated antibodies. Actin was used as a loading control. |
 | Figure 4Effect of ghrelin on the phosphorylation and activity of Akt and Akt-mediated downstream molecules in thymocytes. Cells were stimulated with 100 nM ghrelin for the indicated times. Lysates were assayed by immunoblotting with the indicated antibodies. β-actin was used as the loading control. (A) Tyrosine phosphorylation of Akt, Erk1/2, and p38 upon ghrelin stimulation. (B) Inhibitory effects of Akt inhibitor IV. Cells were pretreated with Akt inhibitor IV for 30 min as indicated, followed by stimulation with 100 nM ghrelin for 15 min. (C) Effects of ghrelin on the phosphorylation of Akt and FoxO3a. (D) Effects of Ghrelin receptor inhibitor ([D-Lys-3]-GHRP-6) on ghrelin-mediated Akt and MAPK activation. Cells were stimulated with 20 µM [D-Lys-3]-GHRP-6 and/or 100 nM ghrelin for 1 hr. |
 | Figure 5Effect of ghrelin on the binding of FoxO3a to 14-3-3 upon restraint stress-induced thymic apoptosis. (A, B) Cell lysates were obtained from control (–STR) versus stressed (+STR) thymus tissues, then immunoprecipitated with FoxO3a (A) or 14-3-3 (B). The immune complexes were subjected to western blot analysis using the indicated antibodies. |
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government (NRF-2014R1A1A1003163).
References
2. Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr Opin Pharmacol. 2010; 10:408–424.

3. Berczi I, Quintanar-Stephano A, Kovacs K. Neuroimmune regulation in immunocompetence, acute illness, and healing. Ann N Y Acad Sci. 2009; 1153:220–239.

4. Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008; 84:915–923.

5. Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PLoS One. 2011; 6:e17940.

6. Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994; 152:5014–5021.
7. Lee JH, Patel K, Tae HJ, Lustig A, Kim JW, Mattson MP, Taub DD. Ghrelin augments murine T-cell proliferation by activation of the phosphatidylinositol-3-kinase, extracellular signal-regulated kinase and protein kinase C signaling pathways. FEBS Lett. 2014; 588:4708–4719.

8. Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011; 340:44–58.

9. Prodam F, Filigheddu N. Ghrelin gene products in acute and chronic inflammation. Arch Immunol Ther Exp (Warsz). 2014; 62:369–384.

10. DeBoer MD. The use of ghrelin and ghrelin receptor agonists as a treatment for animal models of disease: efficacy and mechanism. Curr Pharm Des. 2012; 18:4779–4799.

11. Lee JH, Halperin-Sheinfeld M, Baatar D, Mughal MR, Tae HJ, Kim JW, Carter A, Lustig A, Snir O, Lavie G, Okun E, Mattson MP, Sredni B, Taub DD. Tellurium compound AS101 ameliorates experimental autoimmune encephalomyelitis by VLA-4 inhibition and suppression of monocyte and T cell infiltration into the CNS. Neuromolecular Med. 2014; 16:292–307.

12. Tesauro M, Schinzari F, Caramanti M, Lauro R, Cardillo C. Cardiovascular and metabolic effects of ghrelin. Curr Diabetes Rev. 2010; 6:228–235.

13. Zhang GG, Cai HQ, Li YH, Sui YB, Zhang JS, Chang JR, Ning M, Wu Y, Tang CS, Qi YF, Yin XH. Ghrelin protects heart against ERS-induced injury and apoptosis by activating AMP-activated protein kinase. Peptides. 2013; 48:156–165.

14. Baldelli R, Bellone S, Broglio F, Ghigo E, Bona G. Ghrelin: a new hormone with endocrine and non-endocrine activities. Pediatr Endocrinol Rev. 2004; 2:8–14.
16. Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007; 117:2778–2790.

17. El-Missiry MA. Antioxidant Enzyme. Rijeka, Croatia: InTech;2012. p. 303–320.
20. Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003; 73:689–701.

21. Patel K, Dixit VD, Lee JH, Kim JW, Schaffer EM, Nguyen D, Taub DD. The GHS-R blocker D-[Lys3] GHRP-6 serves as CCR5 chemokine receptor antagonist. Int J Med Sci. 2012; 9:51–58.

22. Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002; 159:1029–1037.

23. Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghe C, Isgaard J, Papotti M, Ghigo E, Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3',5'-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology. 2007; 148:512–529.

24. Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, Kim SY, Lee HK, Kim YB, Park KS. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004; 18:2291–2301.

25. Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008; 198:511–521.

26. Bonfili L, Cuccioloni M, Cecarini V, Mozzicafreddo M, Palermo FA, Cocci P, Angeletti M, Eleuteri AM. Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis. 2013; 18:1188–1200.

27. Conconi MT, Nico B, Guidolin D, Baiguera S, Spinazzi R, Rebuffat P, Malendowicz LK, Vacca A, Carraro G, Parnigotto PP, Nussdorfer GG, Ribatti D. Ghrelin inhibits FGF-2-mediated angiogenesis in vitro and in vivo. Peptides. 2004; 25:2179–2185.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download