Abstract
Sirtuin family members with lysine deacetylase activity are known to play an important role in anti-aging and longevity. Cellular senescence is one of the hallmarks of aging, and downregulation of sirtuin is reported to induce premature senescence. In this study, we investigated the effects of small-molecule sirtuin inhibitors on cellular senescence. Various small molecules such as tenovin-1 and EX527 were employed for direct sirtuin activity inhibition. U251, SNB-75, and U87MG glioblastoma cells treated with sirtuin inhibitors exhibited phenotypes with nuclear enlargement. Furthermore, treatment of rat primary astrocytes with tenovin-1 also increased the size of the nucleus. The activity of senescence-associated β-galactosidase, a marker of cellular senescence, was induced by tenovin-1 and EX527 treatment in U87MG glioblastoma cells. Consistent with the senescent phenotype, treatment with tenovin-1 increased p53 expression in U87MG cells. This study demonstrated the senescence-inducing effect of sirtuin inhibitors, which are potentially useful tools for senescence research.
The mammalian sirtuin family comprises seven enzymes (SIRT1–SIRT7). The prototype of sirtuin is a silent information regulator 2 (Sir2) that is found in yeast and has histone deacetylase activity. Of the seven mammalian sirtuin members, SIRT1-3 and SIRT7 have primarily lysine deacetylase activity. The known physiological functions of sirtuins include proliferation, apoptosis, DNA repair, metabolism, and inflammation (1).
Sirtuins have received a lot of attention from the research community owing to the observation that Sir2 prolongs life in yeast, Caenorhabditis elegans, and Drosophila, although conflicting results have been reported in the literature (2). Naturally, sirtuins have become a focus for research into age-related diseases and anti-aging. Cellular senescence, referring the irreversible arrest of cell growth, is one of the hallmarks of aging (3). The causes of cellular senescence include telomere shortening, genomic damage, mitogenic signaling, and activation of tumor suppressors (4). For tumor suppressors that induce cellular senescence, the p53/p21 and p16INK4a/pRB pathways are known to be of major importance (4). Given the relationship between aging and sirtuins, there are accumulating evidences that the downregulation of sirtuins induces premature senescence and accelerates the aging process (5). For example, the downregulation of SIRT1 expression by miR-217 induces cellular senescence in endothelial cells (6). However, the effects of small-molecule inhibitors of sirtuin on cellular senescence have not yet been investigated. Direct evidences for associating sirtuin activity and cellular senescence are required.
The majority of small-molecule sirtuin modulators are sirtuin activators that have been developed for the purpose of metabolic and neurodegenerative disease therapy (7). Several sirtuin inhibitors have been discovered in the search for anti-cancer agents (8). Specific sirtuin inhibitors characterized so far include EX527 (9), sirtinol (10), cambinol (11), suramin (12), AGK2 (13), tenovins (14), salermide (15), JGB1741 (16), inauhzin (17), and toxoflavin (18). In this study, we investigated the effect of sirtuin inhibitors on the cellular senescence phenotype. The nuclei of glioblastoma cells and primary astrocytes were enlarged and exhibited increased senescence-associated β-galactosidase (SA-β-gal) activity.
The U87MG human glioblastoma cell line and the A549 lung cancer cell line were purchased from the Korean Cell Line Bank (Seoul, Korea). U251, SNB-75, SF295, and XF-498 cell lines were obtained from the Developmental Therapeutics Program (DTP) and Division of Cancer Treatment and Diagnosis (DCTD) Tumor Repository at the National Cancer Institute (Frederick, MD, USA). The cells were cultured in RPMI medium (Sigma-Aldrich Corp., St. Louis, MO, USA) supplemented with 10% FBS and 1% penicillin/streptomycin. The cultured cells were incubated at 37℃ in a humidified 5% CO2 incubator.
Rat primary astrocytes were cultured from the frontal cortices of 2-day-old Sprague-Dawley rat pups. Cortices were dissected out and digested with trypsin to isolate single cells. The cells were cultured in DMEM/F12 media containing 10% FBS for 7 days and then re-plated after trypsin-EDTA digestion. The purity of astrocyte culture was more than 95% as determined by immunostaining with antibodies against the astrocyte-specific marker glial fibrillary acidic protein (GFAP).
The cells were seeded onto an 8-well culture slide and treated with drugs as indicated. The cells were fixed with 4% formaldehyde solution and then permeabilized with 0.5% Triton X-100 solution. The cells were then stained with 50 ng/mL DAPI (Sigma-Aldrich Corp.) and visualized using an Axio Observer.Z1 fluorescence microscope (Carl Zeiss, Germany). Nuclear size was measured using ImageJ software (National Institute of Health, Bethesda, MD, USA).
The cells were cultured in a 6-well plate and SA-β-gal staining was performed using a senescence β-galactosidase staining kit (Cell Signaling Technology, Danvers, MA, USA). The cells were treated with a fixative solution for 15 min at room temperature and washed twice with PBS. The cells were then stained with β-galactosidase staining solution including X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) and staining solution supplement. The plate was incubated at 37℃ overnight. Cells were observed under an Axiovert 200 inverted microscope (Carl Zeiss) at 200× magnification.
Whole cell lysates were lysed with SDS lysis buffer (12 mM Tris-HCl, pH 6.8, 5% glycerol, 0.4% SDS) and subjected to electrophoresis on an SDS-polyacrylamide gel followed by western blotting. The proteins separated on the gel were transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). Antibodies against acetylated-tubulin (Ac-tubulin; mouse monoclonal IgG; T6793, 1:1,000) and β-actin (mouse monoclonal IgG; A5441, 1:5,000) were obtained from Sigma-Aldrich Corp. p53 (mouse monoclonal IgG; sc-98, 1:1,000) antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-mouse IgG (#115-035-033; 1:5,000) secondary antibodies were from Jackson ImmunoResearch Laboratories, Inc., (West Grove, PA, USA). Blots were developed using an Amersham ECL Select chemiluminescent substrate (GE Healthcare, UK), and luminescent signals were visualized using a LAS mini 4000 imager (GE Healthcare).
Small-molecule inhibitors of sirtuin activity were employed to investigate the role of sirtuin in glioblastoma cells. Tenovin-1, known to inhibit both SIRT1 and SIRT2 activity (14), and EX527, reported to inhibit SIRT1 selectively (9), were used to treat glioblastoma cell lines. U251 and SNB-75 cells were treated with 5 µM of tenovin-1 and EX527, respectively, for 48 h, and the cell nuclei were stained with DAPI. DMSO, vehicle for solubilizing tenovin-1 and EX527, was used as negative control. As shown in Fig. 1, the nuclei in the sirtuin inhibitor-treated cells were bigger than those in the DMSO-treated cells. To quantify this phenotype, the DAPI-stained area was measured using ImageJ software. The average pixel sizes of the DAPI-stained areas are shown in Table I. Tenovin-1 treatment increased the size of the nuclei by 1.9-fold in the U251 cells and by 1.4-fold in the SNB-75 cells. Treatment with EX527 also enlarged the U251 and SNB-75 cell nuclei (Table I). Other glioblastoma cell lines were tested for the effect of tenovin-1 and EX527. The size of the nuclei in U87MG and XF498 cells was also affected by tenovin-1 treatment (Supplemental Figure 1). In contrast, A549 lung cancer cells were not affected by tenovin-1 treatment (Supplemental Figure 2).
Sirtuin inhibitors other than tenovin-1 and EX527 were tested. Sirtinol, suramin, salermide, and toxoflavin were used to determine whether the senescence-inducing effect is mediated by sirtuin inhibition. All the small-molecule sirtuin inhibitors tested increased the size of the nucleus in U87MG cells (Table II). These data indicate that inhibition of sirtuin activity by small molecules can affect the size of the nucleus in glioblastoma cells.
The effect of sirtuin inhibitors was investigated in rat primary astrocytes. Primary astrocytes were prepared and cells were treated with tenovin-1, as indicated in Fig. 2A. After DAPI staining, the nuclei were measured. The number of cells with nuclei bigger than those in the control group were counted using ImageJ software. As shown in the Fig. 2A graph, we observed a 5-fold increase in the number of cells with enlarged nuclei. Astrocytes activated with LPS exhibited a similar result when treated with 5 µM tenovin-1 (Fig. 2B). In both the serum-free condition and the serum-supplemented condition, tenovin-1 treatment increased the number of cells with enlarged nuclei. LPS treatment alone did not have a statistically significant effect on the nuclear size. At the condition of LPS activation and serum-free environment, tenovin-1 induced enlargement of nuclear size in primary astrocytes.
Nuclear enlargement is one of the phenotypes presented by senescent cells (1920), and sirtuin is known to have an important function in aging (8). However, the effect of small-molecule sirtuin inhibitors on senescence has not yet been reported. Thus, 5 µM tenovin-1 and 5 µM EX527 were used to treat U87MG and SF295 cells for 24 h, and an SA-β-gal assay was carried out. As shown in Fig. 3A and 3B, the cells treated with tenovin-1 or EX527 showed higher SA-β-gal activity than the DMSO-treated cells (the control). Tenovin-1 exhibits cytotoxicity against U87MG cells, whereas EX527 does not affect cell viability. Both tenovin-1 and EX527 induced higher SA-β-gal activity, as shown in Fig. 3A and 3B. Thus, cellular senescence induced by tenovin-1 or EX527 does not appear to be related to cell death.
Next, the expression levels of acetylated tubulin and p53 were measured. Tenovin-1 activates p53 by inhibiting the deacetylation activity of SIRT1 and SIRT2 (14), and tubulin is a substrate of SIRT2 (21). Furthermore, p53 overexpression arrests senescence (4). In the U87MG cells, treatment with tenovin-1 increased the levels of acetylated tubulin and p53 in a dose-dependent manner (Fig. 3C), consistent with previous reports. Upregulation of p53 by tenovin-1 treatment may be associated with the senescence phenotype.
Although no single characteristic is exclusive to senescent cells, adherent cells exhibiting senescence are generally enlarged and flattened (4). SA-β-gal is commonly used as a marker for senescence (4). Nuclear enlargement has been observed in senescent human fibroblasts. Mitsui and Schneider first observed increased nuclear sizes in human fibroblasts at 1976 (19). Kobayashi et al. also reported nuclear swelling in normal human fibroblasts (20). Report by Sadaie et al. employed morphological changes in nuclei of human fibroblast to screen small molecules affecting senescence (22). In this study, we found that tenovin-1 and EX527 treatment induced SA-β-gal activity (Fig. 3), and we noticed nuclear enlargement in glioblastoma cells and primary astrocytes. These observations indicate that small-molecule sirtuin inhibitors induce cellular senescence in CNS cells.
Nuclear enlargement does not appear to be a universal phenotype for senescence. Among the cell types reported to exhibit increased nuclear size with senescence are human diploid fibroblasts (1920). The same phenotype was observed in glioblastoma cells and primary astrocytes in this study. Apparently, not all cell types share the enlarged nucleus phenotype. The A549 lung cancer cells treated with tenovin-1 did not show an increase in nuclear size (Supplemental Figure 2). Currently, it is not clear whether other kinds of cell share this cellular senescence phenotype, owing to lack of data. Also it is not known why there are discrepancies in nuclear phenotype between cell types. Nevertheless, nuclear enlargement may be a characteristic of cellular senescence in CNS cells and human diploid fibroblasts.
The relationship between sirtuins and aging has been known for decades (5), and the importance of SIRT1 in cellular senescence and premature aging has been acknowledged. However, to the best of our knowledge the senescence effects of sirtuin inhibitors have not been investigated previously. Based on the anti-aging effects by SIRT1, sirtuin inhibitors are expected to expedite aging and senescence at the cellular level. As expected, various sirtuin inhibitors were shown to induce cellular senescence in this study (Table II). Tenovin-1 is reported to have cytotoxic effect (14), and EX527 does not affect cell proliferation (data now shown). Regardless of the presence or absence of cytotoxic effect, the chemicals we tested all show nuclear enlargement phenotype (Table II).
Senescence is an important phenomenon in diseases such as cancer as well as in aging. From that perspective, this study will provide useful tools for the research of senescence. Furthermore, this research has reinforced our understanding of the relationship between sirtuin and senescence.
Figures and Tables
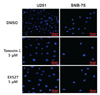 | Figure 1Effect of sirtuin inhibitors on nuclear size in glioblastoma cells. U251 and SNB-75 glioblastoma cells were treated with DMSO (control), 5 µM tenovin-1, or 5 µM EX527 for 48 h, as indicated. The cells were stained with DAPI and the size of the nucleus was measured as described in the Materials and Methods section. |
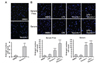 | Figure 2Effect of sirtuin inhibitors on nuclear size in rat primary astrocytes. (A) Rat primary astrocytes were treated with DMSO (control) and 5 µM tenovin-1 for 48 h, as indicated. The cells were stained with DAPI and the number of cells with a nucleus larger than 12.8 µm (the average nuclear size in the DMSO-treated group) was determined (** indicates a p-value<0.01). (B) Rat primary astrocytes were treated with DMSO (control), 0.1 µg/mL LPS, 5 µM tenovin-1, or LPS and 5 µM tenovin-1 for 48 h. The cells were stained with DAPI and the number of cells with a nucleus larger than 12.8 µm was determined (** indicates a p-value < 0.01). |
 | Figure 3Effect of sirtuin inhibitors on the senescence markers. (A) U87MG glioblastoma cells and (B) SF295 cells were treated with DMSO (control), 5 µM tenovin-1, and 5 µM EX527 for 24 h. Senescence-associated β-galactosidase (SA-β-gal) activity was then measured according to the manufacturer's protocol. (C) U87MG cells were treated with tenovin-1 with the indicated concentration. Cell lysates were then subjected to western blotting using antibodies against acetylated tubulin (Ac-tubulin), p-53, and β-actin. |
Table I
Enlargement of nuclear size by sirtuin inhibitor treatment in glioblastoma cells

| Cell line | DMSO | Tenovin-1 | EX527 |
|---|---|---|---|
| U251 | 846.10 | 2047.52* | 6618.31* |
| SNB-75 | 3387.53 | 5230.94** | 4348.06** |
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A2A01053928), and by the Gyeongsang National University Fund for Professors on Sabbatical leave, 2016.
References
1. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010; 5:253–295.

3. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–1217.

4. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013; 75:685–705.
5. Ghosh S, Zhou Z. SIRTain regulators of premature senescence and accelerated aging. Protein Cell. 2015; 6:322–333.

6. Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009; 120:1524–1532.

7. Stunkel W, Campbell RM. Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen. 2011; 16:1153–1169.

8. Carafa V, Nebbioso A, Altucci L. Sirtuins and disease: the road ahead. Front Pharmacol. 2012; 3:4.

9. Napper AD, Hixon J, McDonagh T, Keavey K, Pons JF, Barker J, Yau WT, Amouzegh P, Flegg A, Hamelin E, Thomas RJ, Kates M, Jones S, Navia MA, Saunders JO, DiStefano PS, Curtis R. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem. 2005; 48:8045–8054.

10. Mai A, Massa S, Lavu S, Pezzi R, Simeoni S, Ragno R, Mariotti FR, Chiani F, Camilloni G, Sinclair DA. Design, synthesis, and biological evaluation of sirtinol analogues as class III histone/protein deacetylase (Sirtuin) inhibitors. J Med Chem. 2005; 48:7789–7795.

11. Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S, Kollipara R, Depinho RA, Gu Y, Simon JA, Bedalov A. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 2006; 66:4368–4377.

12. Trapp J, Meier R, Hongwiset D, Kassack MU, Sippl W, Jung M. Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins). ChemMedChem. 2007; 2:1419–1431.

13. Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007; 317:516–519.

14. Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M, McCarthy A, Appleyard V, Murray KE, Baker L, Thompson A, Mathers J, Holland SJ, Stark MJ, Pass G, Woods J, Lane DP, Westwood NJ. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008; 13:454–463.

15. Lara E, Mai A, Calvanese V, Altucci L, Lopez-Nieva P, Martinez-Chantar ML, Varela-Rey M, Rotili D, Nebbioso A, Ropero S, Montoya G, Oyarzabal J, Velasco S, Serrano M, Witt M, Villar-Garea A, Imhof A, Mato JM, Esteller M, Fraga MF. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009; 28:781–791.

16. Kalle AM, Mallika A, Badiger J, Alinakhi , Talukdar P, Sachchidanand . Inhibition of SIRT1 by a small molecule induces apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2010; 401:13–19.

17. Zhang Q, Zeng SX, Zhang Y, Zhang Y, Ding D, Ye Q, Meroueh SO, Lu H. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol Med. 2012; 4:298–312.

18. Choi G, Lee J, Ji JY, Woo J, Kang NS, Cho SY, Kim HR, Ha JD, Han SY. Discovery of a potent small molecule SIRT1/2 inhibitor with anticancer effects. Int J Oncol. 2013; 43:1205–1211.

19. Mitsui Y, Schneider EL. Increased nuclear sizes in senescent human diploid fibroblast cultures. Exp Cell Res. 1976; 100:147–152.

20. Kobayashi Y, Sakemura R, Kumagai A, Sumikawa E, Fujii M, Ayusawa D. Nuclear swelling occurs during premature senescence mediated by MAP kinases in normal human fibroblasts. Biosci Biotechnol Biochem. 2008; 72:1122–1125.

21. North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+- dependent tubulin deacetylase. Mol Cell. 2003; 11:437–444.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download