Abstract
Programmed death-1 (PD-1) is a strong negative regulator of T lymphocytes in tumor-microenvironment. By engaging PD-1 ligand (PD-L1) on tumor cells, PD-1 on T cell surface inhibits anti-tumor reactivity of tumor-infiltrating T cells. Systemic blockade of PD-1 function using blocking antibodies has shown significant therapeutic efficacy in clinical trials. However, approximately 10 to 15% of treated patients exhibited serious autoimmune responses due to the activation of self-reactive lymphocytes. To achieve selective activation of tumor-specific T cells, we generated T cells expressing a dominant-negative deletion mutant of PD-1 (PD-1 decoy) via retroviral transduction. PD-1 decoy increased IFN-γ secretion of antigen-specific T cells in response to tumor cells expressing the cognate antigen. Adoptive transfer of PD-1 decoy-expressing T cells into tumor-bearing mice potentiated T cell-mediated tumor regression. Thus, T cell-specific blockade of PD-1 could be a useful strategy for enhancing both efficacy and safety of anti-tumor T cell therapy.
Cancer Immunotherapy targeting enhancement of anti-tumor T cell reactivity has received significant attention in recent years, mainly due to the favorable clinical responses of immune checkpoint inhibitors, such as anti-CTLA4 and anti-PD-1 antibodies (1). Anti-PD-1 antibodies in particular showed significant objective responses in clinical trials for some solid tumors, such as malignant melanoma (30%), non-small cell lung cancer (NSCLC; 20%), renal cell carcinoma (17%), and etc (2345). To this end, two PD-1 antibody drugs, nivolumab and pembrolizumab, have received US FDA approval for treatment of advanced melanoma, NSCLC, and metastatic renal cell carcinoma. Also, antibodies against PD-L1, a ligand for PD-1, are being tested in clinical trials for various types of cancers (6). Thus, therapeutic strategies targeting the PD-1/PD-L1 axis have considerable potential to contribute to the treatment of multiple types of tumors.
PD-1 is an inhibitory receptor expressed mainly on activated T cells, B cells and monocytes (78). PD-L1 and PD-L2 are best-known ligands for PD-1 (910). Both are expressed on hematopoietic cells (dendritic cells, mast cells, macrophages, T cells and B cells), whereas PD-L1 is also expressed on non-hematopoietic cells (endothelial and epithelial cells) (1112). Interestingly, PD-L1 expression is increased on various types of cancer cells, suggesting that this molecule is used for immunoresistance of cancer cells (13). Biochemically, PD-1 delivers inhibitory signal through its intracellular cytoplasmic domain, which contains immunoreceptor tyrosine-based switch motif (ITSM) and immunoreceptor tyrosine-based inhibitory motif (ITIM). Ligation of PD-L1 with PD-1 induces phosphorylation of ITSM and ITIM and recruits SHP-1 and -2, which inhibits T cell receptor downstream signaling (1415).
In murine in vivo studies, PD-1 deficiency led to the development of autoimmune diseases such as lupus-like syndrome and dilated cardiomyopathy (1617). Therefore, one can expect that systemic treatment with PD-1 blocking antibodies in cancer patients will lead to autoimmune side effects. Indeed, 10 to 15% of treated patients developed grade 3~4 drug-related toxicities, although these toxicities were less severe than those of blocking antibodies against CTLA4, another co-inhibitory receptor on T cells (181920).
In this study, to utilize PD-1 blockade in a T-cell specific manner rather than systemically, we tried to inhibit endogenous PD-1 function in T cells by overexpressing a PD-1 mutant on T cells that is designed compete with endogenous PD-1 in a dominant negative manner. The mutant receptor was generated by deleting the cytoplasmic domain of PD-1, which we call PD-1 decoy. T cells expressing PD-1 decoy showed increased production of IFN-γ when co-cultured with PD-L1 expressing tumor cells in vitro and showed increased tumor regression in vivo. Thus, inhibiting PD-1 function in tumor-specific T cells will be a beneficial strategy for enhancing T cell-mediated anti-cancer therapy without the systemic toxicity induced by PD-1 blockade.
B6, OT-I and Pmel-1 mice were obtained from the Jackson laboratories. The mice were maintained in a specific pathogen-free facility at the Research Institute National Cancer Center, Korea in accordance with the guidelines of the Institutional Animal Care and Use Committee. E.G7 lymphoma cells and B16-F10 (B16) melanoma cells were purchased from ATCC. Phoenix Eco and Phoenix GP cell lines were provided by Garry Nolan (Stanford University).
The cDNA of extracellular and trans-membrane portion of mouse PD-1 (amino acid 1-199) was cloned into pMIG-w retroviral expression vector (a gift from Yosef Refaeli, National Jewish Medical and Research Center) to generate PD-1 decoy. For PD-1-CD28 chimera, the cDNA of the cytoplasmic portion of mouse CD28 (amino acid 177~218) was linked to the cDNA of extracellular and trans-membrane portion of PD-1 (amino acid 1~199) by blunt end ligation. PD-1 decoy and PD-1-CD28 chimera cDNAs were inserted in front of the IRES-GFP cassette in pMIG-w to use GFP as an expression marker. All sequences were confirmed by automated DNA sequencing.
Phoenix GP cells were transiently transfected with a VSV-G (pMD.G)-coding plasmid and the retroviral plasmids by calcium- phosphate transfection. After 48 hours, the cultured supernatant containing VSV-G pseudotyped retrovirus was harvested. Subsequently, Phoenix Eco cells were transduced with the supernatant overnight. After 3~5 days, GFPhi populations were sorted by flow cytometry to generate stable cell lines producing the retrovirus. The supernatants harvested from the stable cell lines were concentrated 10 fold using a 100 kDa cut-off centricon (Millipore) to increase T cell-transduction efficiency. B6 splenocytes were activated with anti-CD3 antibody (5 µg/ml) plus anti-CD28 antibody (2 µg/ml) for 24 hours. Splenocytes from OT-I and Pmel-1 mice were activated with OVA257-264 peptide (1 µM) or hgp100 peptide (1 µM), respectively. The activated T cells were transduced with the concentrated retroviral supernatant plus polybrene (6 mg/ml) by spin infection. After 48 hours, the transduced T cells were rested for 3 days in the presence of recombinant human IL-2 (30 U/ml) without further stimulation. More than 95% of live cells were T cells after the resting. Transduction efficiency was measured by GFP positivity using flow cytometry. PE-conjugated anti-mouse PD-1 antibody (Clone: J43, BD Bioscience) was used to detect PD-1 decoy, PD-1-CD28 chimera, and endogenous PD-1.
GFP positive retrovirus-transduced B6 splenocytes were sorted by flow cytometry. GFPhi populations were composed of 60~70% of CD8 T cells and 25~35% of CD4 T cells before cell sorting. The sorted T cells (2×104) were stimulated with indicated amount of anti-CD3 antibody in the presence of irradiated splenocytes (2×105) for 48 hours followed by IFN-γ ELISA. Retrovirus-tranduced OT-I cells (serial dilution from 105 to 102 cells) were cultured with MC38-OVA cells (1×104) or E.G7-OVA cells (1×105) for 24 hours. The cultured supernatants were harvested and IFN-γ was measured by ELISA. For testing the efficacy of PD-1-CD28 chimera, Pmel-1 cells were transduced and co-cultured with IFN-γ (20 ng/ml)-treated B16 melanoma cells for 48 hours followed by IFN-γ ELISA.
E.G7 cells (2×106) were subcutaneously injected into B6 mice on day 0. After 7 days, the retrovirus-transduced OT-I cells (2×106) were adoptively transferred into the tumor-bearing mice via intravenous injection. Tumor growth was measured every 3 to 4 days from day 7 until mice were euthanized. The approximate tumor sizes were calculated using the following formula: length×width×π (mm2). When tumor sizes exceed 500 mm2, the mice were euthanized. Statistical comparisons were made using the Wilcoxon matched pairs test.
In order to generate a dominant negative mutant of PD-1, we designed a deletion mutant of PD-1, PD-1 decoy, which includes the extracellular and transmembrane domain of PD-1 with its intracellular domain deleted. This design allows PD-1 decoy to bind the ligand, but prevents it from delivering inhibitory signals inside the cell. Therefore, this mutant receptor is expected to compete with endogenous PD-1 for ligand binding and inhibit endogenous PD-1 function. To overexpress PD-1 decoy on T cells, we constructed a retroviral expression vector of this receptor. Retrovirus-transduced T cells were identified by GFP expression since the retroviral vector contains GFP cDNA as a reporter. When activated mouse splenic T cells were transduced with the retrovirus, transduction efficiency was approximately 65%, as measured by GFP positivity. GFP-positive T cells transduced with a PD-1 decoy-encoding virus were more strongly stained with anti-PD-1 antibody than those transduced with empty virus, which ensured overexpression of transduced PD-1 decoy (Fig. 1A). We hypothesized that overexpressed PD-1 decoy would diminish the co-inhibitory function of endogenous PD-1 and enhance functional activation of T cells. To test this idea, we sorted GFP-positive T cells via flow cytometry and stimulated them with anti-CD3 in the presence of irradiated splenocytes. When we measured secreted IFN-γ in the culture supernatant, PD-1 decoy-expressing T cells produced 2~3 fold more cytokines than control T cells (Fig. 1B). Therefore, it is very likely that PD-1 decoy interrupts binding of endogenous PD-1 to PD-1 ligands on splenocytes and inhibits endogenous PD-1 function.
Next, we examined whether this functional enhancement of T cells could be applied to the anti-tumor reactivity of tumor-specific T cells. For this purpose, we utilized ovalbumin (OVA) as a model tumor antigen. OVA-transfected tumor cell lines and OVA-specific TCR-transgenic CD8 T cells (OT-1) served as target tumors and tumor-specific T cells respectively. OT-I T cells transduced with the PD-1 decoy-encoding retrovirus showed 55~65% transduction efficiency (GFP positivity), and the level of PD-1 decoy expressed in the GFP-positive population was higher than that of endogenous PD-1 expressed in the empty-vector-transduced control cells (Fig. 2A). For OVA-expressing tumor cell lines, we chose MC38-OVA, mouse colon cancer cells transfected with OVA, and E.G7, a mouse lymphoma cell line transfected with OVA because both cell lines express PD-L1, a ligand of PD-1, on their cell surface (Fig. 2B). When PD-1 decoy-transduced OT-1 T cells were incubated with these cell lines, they produced a higher amount of IFN-γ than control OT-1 T cells (Fig. 2C). The degree of enhancement of IFN-γ production was higher for E.G7-stimulated T cells than that for MC-38-OVA-stimulated T cells, which was correlated with higher PD-L1 expression on E.G7 cells than that on MC-38-OVA. Hence, PD-1 decoy improves the reactivity of tumor antigen- specific T cells in response to PD-L1-expressing tumor cells.
In our previous study on CTLA4, another inhibitory receptor on T cell surface, we reported that replacing the inhibitory cytoplasmic domain of CTLA4 with the stimulatory cytoplasmic domain of CD28 resulted in conversion of the negative signal of CTLA4 to a positive signal (21). Thus, overexpression of this chimeric receptor on tumor-specific T cells led to enhanced T-cell mediated tumor regression owing to the dominant negative effect on endogenous CTLA4 as well as the additional surrogate CD28 costimulatory signal. In this study, we also tested if a similar substitution of the CD28 cytoplasmic domain for the cytoplasmic domain of PD-1 would further increase T cell reactivity by providing additional costimulation. For this experiment, we generated a PD-1-CD28 chimera that consists of the extracellular and transmembrane domain of PD-1 and the intracellular cytoplasmic domain of CD28 (Fig. 3A). In this case, we used another tumor antigen-specific TCR transgenic (Pmel-1) CD8 T cells which recognize gp100 melanoma antigen. As a target tumor cell line, B16 melanoma cells were used. Although B16 cells showed moderate levels of PD-L1 expression on the cell surface, IFN-γ treatment further enhanced PD-L1 expression (Fig. 3B). Therefore, we incubated Pmel-1 T cells transduced with PD-1 decoy or PD-1-CD28 chimera virus with IFN-γ-treated B16 cells, and measured IFN-γ production from this co-culture. Similar to the results from OT-1 T cells, PD-1 decoy-transduced Pmel-1 T cells produced a higher amount of IFN-γ than control cells. PD-1-CD28 chimera also showed an enhancing effect on IFN-γ production from Pmel-1 T cells. However, it did not further enhance IFN-γ production from Pmel-1 T cells when compared with PD-1 decoy (Fig. 3C). This observation indicates that this chimera only exerted its dominant negative function without further costimulatory activity. This result is in stark contrast to a previous report that a PD-1-CD28 chimera in humans delivered CD28 signal in a CD28-null lymphoma cell line (22). This discrepancy may be due to slight differences in construct design between the two chimeras. Our chimera used the transmembrane domain of PD-1 molecule and the other used the transmembrane domain of CD28. Structurally, PD-1 exists as a monomer, whereas CD28 functions as a dimer. Thus, the CD28 domain in our construct may not have been able to dimerize to deliver its activation signal. In contrast, the previously published construct can dimerize because it has the transmembrane and cytoplasmic domain of CD28, which can be linked by disulfide bonds. This dimerization did not seem to affect the binding of this molecule to PD-L1. Nonetheless, our result showed that the inhibition of endogenous PD-1 signals with PD-1 decoy alone was sufficient to enhance the functional activity of tumor-specific T cells.
Finally, we investigated whether PD-1 decoy-mediated functional enhancement of T cells could increase therapeutic efficacy in vivo using a mouse model of anti-tumor T cell therapy. OT-1 T cell-mediated regression of OVAexpressing tumors is such a model. When OT-1 T cells were adoptively transferred to subcutaneous E.G7 tumor-bearing mice, tumor growth was significantly reduced. PD-1 decoy overexpression in OT-1 T cells greatly potentiated tumor regression in this model, consistently with the results from in vitro functional studies (Fig. 4). This result demonstrates that functional blockade of PD-1 in adoptively transferred tumor-specific T cells without systemic blockade of PD-1 may be a good strategy to enhance the efficacy of T cell therapy and to bypass potential side effect of autoimmunity at the same time.
Figures and Tables
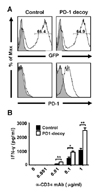 | Figure 1Overexpression of PD-1 decoy increases IFN-γ secretion from T cells. Activated B6 splenic T cells were transduced with retrovirus carrying either a control vector (pMIG-w) or PD-1 decoy and rested for 3 days in the absence of stimulation. (A) Retroviral transduction efficiency was measured by flow cytometry using GFP. The GFP positive populations were gated and the expression levels of PD-1 were analyzed. PD-1 expression in the control represents the levels of endogenous PD-1, while the PD-1 decoy sample shows the levels of both endogenous and the decoy receptor. (Filled gray area: Isotype control, Percentage of GFP positive cells indicated inside histograms) (B) GFP positive T cells were sorted and stimulated with anti-CD3 in the presence of irradiated splenocytes for 48 hours. IFN-γ in the cultured supernatants was measured by ELISA (Student's t-test, *p<0.05, **p<0.01). Results are representative of 3 independent experiments. |
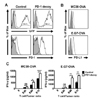 | Figure 2Enhanced anti-tumor reactivity of OT-I T cells carrying PD-1 decoy. OVA-specific CD8 T cells (OT-I cells) were transduced with retroviruses carrying control vector or PD-1 decoy as described in the methods and materials. (A) Transduction efficiency and PD-1 decoy expression of OT-I cells were measured as described in Fig. 1A. (B) Expression of PD-L1 on OVA-expressing tumor cell lines was analyzed by flow cytometry. (A-B, Gray filled area: Isotype control, Percentage of GFP positive cells indicated inside histograms) (C) The retrovirus-transduced OT-I cells were co-cultured with OVA-expressing tumor cell lines for 24 hours, and IFN-γ in the cultured supernatants was measured by ELISA (Student's t-test, ns; not significant, *p<0.05, **p<0.01, ****p<0.0001). The transduced OT-I cells were rested for 3 days in the absence of stimulation and sorted by GFP expression before co-culture with the tumor cells. Results are representative of 2~3 independent experiments (A-C). |
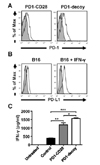 | Figure 3The PD-1-CD28 chimera does not improve the anti-tumor reactivity of T lymphocytes compared to PD-1 decoy. B16 melanoma antigen (gp100)-specific CD8 T cells (Pmel-1 cells) were transduced with retroviruses carrying PD-1-CD28 chimera or PD-1 decoy. (A) Expression levels of PD-1-CD28 chimera and PD-1 decoy in GFP-positive Pmel-1 populations were analyzed by flow cytometry. (B) B16 melanoma cells were either left untreated or treated with IFN-γ (20 ng/ml) for 48 hours. Then, PD-L1 expression was determined by flow cytometry. (C) Retrovirus-transduced GFP positive Pmel-1 cells were sorted by flow cytometry. The sorted cells (1×105) were co-cultured with IFN-γ-treated B16 melanoma cells (1×104) for 48 hours. IFN-γ in the cultured supernatants was quantified by ELISA (Student's t-test, *p<0.05, **p<0.01, ***p<0.001). |
 | Figure 4PD-1 decoy potentiates anti-tumor therapeutic efficacy of adoptively transferred T cells. B6 mice were injected with OVA-expressing E.G7 cells (2×106) subcutaneously. After 7 days, the mice were either left untreated or intravenously injected with control vector (pMIG-w)-transduced (2×10 6) or PD-1 decoy-transduced (2×106) OT-I cells. The retrovirus-transduced OT-I cells were rested for 3 days before adoptive transfer. The mean tumor size of 10 mice per group was recorded (*p=0.0039, Wilcoxon matched-pairs test). Results are representative of 2 independent experiments. |
ACKNOWLEDGMENTS
We thank Drs. Yosef Refaeli and Garry Nolan for providing us valuable materials. This work was supported by grant from Basic Science Research Program through the
National Research Foundation of Korea (NRF) funded by
the Ministry of Science, ICT & Future Planning (NRF-2013R1A2A2A01009444), Republic of Korea.
References
1. Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014; 588:368–376.

2. Henick BS, Herbst RS, Goldberg SB. The PD-1 pathway as a therapeutic target to overcome immune escape mechanisms in cancer. Expert Opin Ther Targets. 2014; 18:1407–1420.

3. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454.

4. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014; 32:1020–1030.

5. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013; 369:134–144.

6. Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015; 27:39–46.

7. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007; 19:813–824.

8. Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996; 8:765–772.

9. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999; 5:1365–1369.

10. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001; 2:261–268.

11. Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002; 84:57–62.

12. Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003; 33:2706–2716.

13. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8:793–800.

14. Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004; 173:945–954.

15. Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001; 98:13866–13871.

16. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999; 11:141–151.

17. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001; 291:319–322.

18. Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets. 2015; 19:201–211.

19. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015; 26:2375–2391.

20. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012; 30:2691–2697.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download