Abstract
To find the relation between exercise and cytokines, we examined the effect of the training intensity on the levels of cytokines, including interferon-gamma (IFN-γ), interlukine-4 (IL-4) and interlukine-4/interferon-gamma ratio (IL-4/IFN-γ ratio) in female Futsal players. Twelve well-trained female college Futsal players aged 19~22 participated in this study. The athletes completed 30-min of running at 60~65% maximal heart rate [moderate-intensity exercise], and 30-min of running at 75~80% maximal heart rate [high-intensity exercise]. peripheral blood samples were collected 24 h before and 24 h and 48 h after each of the exercise bouts. finding showed that The 30-min bout of moderate-intensity exercise induced a significant increase in IFN-γ (p=0.01) and significant decreases in IL-4 (p=0.001) and IL-4/IFN-γ ratio (p=0.003). And also, 30-min of running at 75~80% maximal heart rate induced increase in IFN-γ (p=0.07) and decreased in IL-4 (p=0.01) and IL-4/IFN-γ ratio (p=0.06) that these changes not significantly. In summary, exercise intensity can effect on the magnitude of changes in cytokines. It seems that moderate intensity exercise enhances cytokine pattern in female college Futsal players.
As key health effects associated with exercise can be both beneficial (e.g., reduced risk of cardiovascular disease) and harmful (e.g., exercise-induced asthma), researchers are interested in studying the immune response to exercise. It is known that the human immune system as well as other physiological systems, shows significant changes in response to physical activity (1). These effects may well be mediated by the activity of the immune system parameters (2).
Severe epidemic diseases such as type 2 diabetes, cardiovascular disease, arthritis, obesity and osteoporosis are associated with an increased level of inflammatory cytokines (34). Cytokines, one of the most important products of the immune response, are proteins secreted by immune cells that affect the function of immune cells (5), and also by other cells such as endothelial cells and adiposities (1). Immune responses can be divided into two general categories; Th1 and Th2. Deviation of the immune response toward Th1 can be enhanced cellular immune response and deviation to the Th2 immune response can be enhanced humoral immune response (6). Th1 and Th2 cytokine responses are important that shows the humoral and cellular responses status (5). Th1/Th2 ratio is used as an indicator of changes in the immune system. Interferon gamma and IL-4 are indexes of Th1 and Th2, respectively (7). IFN-γ is secreted by Th1 cells and is involved in cell-mediated immune responses; whereas IL-4 secreted by Th2 cells that regulate humoral immune responses (89). Thus, IL-4/IFN-γ ratio is considered as good measure of immune function (6).
There are several studies were examined the effects of exercise on blood Th1 and Th2 cytokines. Studies showed that cytokine increases in response to exercises while others indicated a decrease (1011). The contradictory results from existing research may in part be explained by the use of a variety of exercise models, subjects, as well as exercise protocols related to intensity and duration. Since gender is a factor affecting the immune system changes (1213), it is also essential to specifically study the immune system effects of various types of exercise in women. Our hypothesis was that exercise can affect the cytokine. Therefore, assuming the exercise intensity can have an effect on the pattern of cytokines; the aim of the study was to investigate the effect of the training intensity on cytokine response in female Futsal players.
Twelve well-trained female college Futsal player between 19 and 22 year participated in this study, which had at least six months Futsal training. All participants were informed about the purpose and risks of the study before written, informed consent was obtained. Subjects' history of heart disease - cardiovascular, hypertension, diabetes, smoking or using drugs not specified. During the study, subjects were asked not to participate in any activities other than training. This study was approved by the local ethical committee of Jahrom University of Medical Sciences (June.rec.1393.059).
The heart rates of all the participants were recorded for the maximal heart rate calculation using Karvonen method (14) as follows:
Once maximal heart rate was determined, the athletes completed on 30-min of running at 60~65% maximal heart rate [moderate-intensity exercise], and 75~80% maximal heart rate [high-intensity exercise] (Table I). During training, maximal heart rate was measured, as described above, every 5 min throughout the trial to ensure that each athlete was exercising at the correct relative intensity. Both trials were conducted between 7:00 a.m. and 9:00 a.m.
During each blood sampling, 2 ml of blood sample were collected from the peripheral blood. The separated sera were tested using commercial ELISA kits for the measurement of serum cytokine concentrations. Serum IL-4 was measured by commercial ELISA kit (Orgenium, Finland, REF: IL04001) follows company instruction. It is based on the direct sandwich technique with biotin-Streptavidin, in which two monoclonal antibodies (Ab) are directed against human IL-4. Human IL-4 present in the sample or standard binds to antibodies adsorbed to the micro wells. A biotin conjugated anti-human IL-4- Ab is added and binds to human IL-4 - captured by the first Ab. Following incubation unbound biotin-conjugated anti-human IL-4 - Ab is removed during a wash step. Streptavidin-HRP is added and binds to the biotin-conjugated anti-human IL-4 Ab. Following incubation unbound Streptavidin-HRP is removed during a wash step, and substrate solution reactive with HRP is added to the wells. A colored product is formed in proportion to the amount of human IL-4 - present in the sample or standard. The reaction is terminated by addition of acid and absorbance is measured at 450 nm. A standard curve is prepared from 7 human IL-4 - standard dilutions and human IL-4 - sample concentration determined. And also, Serum IFN-γ was measured by commercial ELISA kit (BOSTER, USA: Cat. No. EK 0373), According to company instruction as the same method for serum IL-4.
Age, Height, Weight, Body Mass Index, Systolic Blood Pressure, Diastolic Blood Pressure and Heart Rate data of all subjects summarizes in Table II and III.
Moderate-intensity exercise for 30-min induced significant increases in IFN-γ and significant decreases in IL-4 and IL-4/IFN-γ ratio. And also High-intensity exercise for 30-min tended to increase IFN-γ and to decrease IL-4 and IL-4/IFN-γ ratio, but none of the changes reached significance (Table IV).
When analyzed data with a paired t-test we demonstrated that the plasma concentrations of IFN-γ increased (p=0.001) by 44% over 30-min of running at 60~65% maximal heart rate, and a decrease of 34% (p=0.01) was observed for plasma IL-4, altogether resulting in a 74% decrease in IL-4/IFN-γ ratio (Fig. 1, 2 and 3).
This study examined the effects of moderate and high intensity running on the cytokine pattern of female Futsal players. The main findings are that moderate intensity exercise had a significant impact on cytokine pattern.
Interferon gamma level was observed to be significantly elevated during moderate intensity exercises in contrast to high intensity exercise. Previous studies have reported contradictory results in this regards as some researchers reported reduced levels of interferon-gamma (1516), while other scholar reported increased levels of interferon-gamma after performing physical exercises (1017). In addition, it was noted that both high and moderate intensity exercises cause a change in IL-4/IFN-γ ratio, so that moderate intensity exercise resulted in a significant decrease in IL-4/IFN-γ ratio while this decrease was not significant in the high intensity exercises. These findings are consistent with the results of other researchers such as Alberti et al. who argue that physical exercises with moderate to high intensity reduce IL-4/IFN-γ ratio (18). However, the findings of this study are contrary to the results observed by some researchers (151920). These contradictory findings may be attributed to the use of different exercise protocols and may also be related to participant group, their fitness level and exercise model.
IFN-γ is one of the most important cytokines which is secreted by lymphocytes. This cytokine is produced primarily by T lymphocytes and natural killer cells (NK cells) followed by the activation of the immune system and inflammatory stimuli (21). As was mentioned earlier, various reports demonstrate various effects of exercises on levels of cytokines. Previous studies on humans and animals have shown that T cells produce IFN-γ both in vitro and in human body. However, the process may be stopped by cortisol and epinephrine hormones. These hormones (cortisol and epinephrine) increase both during exercises and in response to exercises. In addition, epinephrine affects T cells at the level of antigen presenting cells. It also directly affects T cell receptors (22). Catecholamine may also modulate the immune responses. Although specific cellular responses to catecholamine are dependent on types of changes in adrenergic receptors, catecholamine generally increase cAMP intracellular concentrations and the increased cAMP inhibits the production of IFN-γ (23).
It can be said that IL-4 is a cytokine that plays a significant role in the anti-inflammatory responses. It is known that T cells, mast cells, and neutrophils are the main producers of IL-4. In addition, it has been reported that other cells such as liver, fibroblast, brain, and muscle cells are able to express IL-4 (24). Numerous studies have explored the effect of various sport exercise on IL-4 (252627). Cytokine products may be changed by a set of stimuli, including stress hormones, oxidative stress, and sport exercise (28). A large number of researchers have reported an increase in the concentration of anti-inflammatory cytokines after performing various types of exercises. Research shows that training exercises would increase the concentration of anti-inflammatory cytokines such as IL-4 (2930). Increased concentrations of anti-inflammatory cytokines such as IL-4 has been reported after performing maximal exercises (27), strength exercises (31), downhill running (32), intense cycling (33), endurance running and cycling. In addition, no change was found in plasma levels of IL-4 after a marathon race (34). It has been reported that 6 months of aerobic exercises with different intensities have no effect on plasma levels of IL-4 and IL-10 (35).
Concerning the effects of IL-4, it can be said that it plays a role in muscle hypertrophy and acts as the main inducer of type 2 cytokines and suppress the immune system in the development of tissue injury (24). In response to stress three major systems are activated: the sympathetic nervous system, the parasympathetic nervous system (vagal nerve) and various adrenal glands (36). In contrast, the sympathetic nervous system is most affected by physical stress, such as dynamic exercises (37). Although increased sympathetic activity and secretion of corticosteroids are able to divert the immune system towards Th2 responses (IL-4), the peripheral sympathetic nervous system (because of innervation of the large number of tissues including lymphoid organs) divert immune responses towards theTh2 (IL-4) with more intensity (38).
The balance between Th1 and Th2 immunological responses is very important for maintaining normal immune system.Th1 path primarily acts against intracellular pathogens, especially viruses and bacteria. However, it is believed that the Th2 path acts against intercellular pathogens such as parasites (39). Studies have shown that the performance of sport exercises temporarily increases anabolic/catabolic hormone concentrations (such as testosterone and cortisol) and anti-inflammatory cytokines (30). Experimental evidence suggests that testosterone may inhibit endogenous gene expression of inflammatory cytokines (40), but reinforce anti-inflammatory cytokine gene expression (41). In this regard, some researchers have shown that moderate exercises help the immune system towards type 1 (Th1) cytokine response while vigorous exercises enhance type 2 (Th2) cytokine responses (42). It has been reported that the number of dendritic cells in the blood increases as a result of performing sport exercises. Dendritic cells which activate T cells are themselves activated by IFN-γ. The fact that during moderate exercises the body is diverted towards Th1 (IL-2 and IFN-γ) cytokine responses makes it possible for dendritic cells to respond to exercises as their main function (4344).
Increased level of IFN-γ and reduced IL-4 is an approach that clinicians are trying to achieve it for the treatment of various cancers. Although genetic and immunological factors play an important role in the regulation of Th1 and Th2 cytokine products, it seems that hypothalamic-pituitary-adrenal axis and the sympathetic nervous system play an important role in this approach (45). The results of a study showed that the physical or mental stress can cause adrenal hormone responses, followed by a change in the pattern of IFN-γ and IL-4 production in the spleen and lymph nodes (46). On the other hand, researchers suggest that the synthesis of glucocorticoids, particularly cortisol which is increased as a result of stress prevents the production of IFN-γ and stimulates the production of inflammatory factors such as IL-4. Therefore, it may be said that the circulating concentrations of cortisol can be used for suppressing effects of IFN-γ production and increased IL-4 (46). Stress-related changes in the immune system are affected usually through the activation of the central nervous system and the hypothalamic-pituitary-adrenal axis. The activation of the central nervous system causes the release of catecholamine and causes a delay in the release of glucocorticoids. These hormones change the immune system functions after performing intense exercise and periods of heavy training exercise (47). Catecholamine and glucocorticoids influence on the release of Th cytokines. This is basically done through the stimulation of glucocorticoids and beta 2-adrenergic receptors (48). These stress hormones affect the activity of cellular immunity (Th1) and humeral safety (Th2) (48). In addition, cortisol, adrenaline, and noradrenalin hormones may suppress the release of cytokines from Th1 cells and antigen presenting cells, while positively regulating cytokine production form Th2 cells and diversion towards the production of Th2 cytokines (49).
Based on the results of the study it can be said that physical activities of moderate to high intensity can affect cytokine pattern changes so that the training exercise protocols used can change inflammatory and anti-inflammatory cytokines. In addition, the effects of moderate intensity exercises are higher on IFN-γ-IL-4 ratio than high-intensity exercises. However, different genetic and immunological factors are important in the regulation of Th1 and Th2 cytokine products. Besides, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system seem to play a major role in this regard.
Figures and Tables
 | Figure 1Plasma IFN-γ in female Futsal players before and after 30 min of running at 60~65% maximal heart rate (Moderate), and 75~80% maximal heart rate (High intensity). Data are presented as means±SD. #Denotes significantly different from before training (p<0.05). |
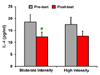 | Figure 2Plasma IL-4 in female Futsal players before and after 30 min of running at 60~65% maximal heart rate (moderate intensity), and 75~80% maximal heart rate (high intensity). Data are presented as means±SD. # Denotes significantly different from before training (p<0.05). |
 | Figure 3Plasma IL-4 - IFN-γ ratio in female Futsal players before and after 30 min of running at 60~65% maximal heart rate (moderate intensity), and 75~80% maximal heart rate (high intensity). Data are presented as means±SD. #Denotes significantly different from before training (p<0.05). |
Table I
Program for the moderate and high intensity exercises

| Intensity | Time | Load | Type of exercise |
|---|---|---|---|
| Moderate-intensity exercise | 30 min | 60~65% maximal heart rate | Running |
| High-intensity exercise | 30 min | 75~80% maximal heart rate | Running |
Table II
Demographic data of all subjects

| SBP (mmHg) | DBP (mmHg) | HR (beats per minute) |
|---|---|---|
| 117.9±8.1 | 72.9±9.4 | 73.3±2.4 |
Table III
Physiological data of all subjects

| Age (yr) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|
| 20.6±1.2 | 164.1±3.4 | 57.50±1.83 | 21.4±1.3 |
Table IV
Changes in IFN-γ, IL-4 and IL-4/IFN-γ ratio immediately before and after 30 min of running at 60~65% maximal heart rate (Moderate), and 75~80% maximal heart rate (High)

References
1. Koch AJ. Immune Response to Exercise. Braz J Biomotricity. 2010; 2:92–93.
2. Cooper DM, Radom-Aizik S, Schwindt C, Zaldivar F Jr. Dangerous exercise: lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol (1985). 2007; 103:700–709.

3. Tuttle HA, Davis-Gorman G, Goldman S, Copeland JG, McDonagh PF. Proinflammatory cytokines are increased in type 2 diabetic women with cardiovascular disease. J Diabetes Complications. 2004; 18:343–351.

4. Moss RB, Moll T, El-Kalay M, Kohne C, Soo HW, Encinas J, Carlo DJ. Th1/Th2 cells in inflammatory disease states: therapeutic implications. Expert Opin Biol Ther. 2004; 4:1887–1896.

5. Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. Saunders Elsevier press;2007. p. 267–303.
6. Shahabi S, Hassan ZM, Mahdavi M, Dezfouli M, Rahvar MT, Naseri M, Jazani NH. Evaluation of the neuroendocrine system and the cytokine pattern in warm and cold nature persons. Physiol Pharmacol. 2007; 11:51–59.
7. Gu X, Li P, Liu H, Li N, Li S, Sakuma T. The effect of influenza virus A on th1/th2 balance and alveolar fluid clearance in pregnant rats. Exp Lung Res. 2011; 37:445–451.

8. Preston JA, Thorburn AN, Starkey MR, Beckett EL, Horvat JC, Wade MA, O'Sullivan BJ, Thomas R, Beagley KW, Gibson PG, Foster PS, Hansbro PM. Streptococcus pneumoniae infection suppresses allergic airways disease by inducing regulatory T-cells. Eur Respir J. 2011; 37:53–64.

9. Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011; 17:673–675.

10. Stefano G, Marco S, Federico A, Davide F, Sergio D. Exercise training lymphocyte subsets and their cytokines production: experience of an Italian professional football team and their impact on allergy. Biomed Res Int. 2014; 2014:429248.
11. Sugama K, Suzuki K, Yoshitani K, Shiraishi K, Kometani T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc Immunol Rev. 2012; 18:116–127.
12. Gillum TL, Kuennen MR, Schneider S, Moseley P. A review of sex differences in immune function after aerobic exercise. Exerc Immunol Rev. 2011; 17:104–121.
13. Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement Part one: Immune function and exercise. Exerc Immunol Rev. 2011; 17:6–63.
14. Jahromi AS, Zar A, Ahmadi F, Krustrup P, Ebrahim K, Hovanloo F, Amani D. Effects of endurance training on the serum levels of tumour necrosis factor-alpha and interferon-gamma in sedentary men. Immune Netw. 2014; 14:255–259.

15. Gholamnezhad Z, Boskabady MH, Hosseini M, Sankian M, Khajavi RA. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran J Basic Med Sci. 2014; 17:1–8.
16. Sellar CM, Syrotuik DG, Field CJ, Bell GJ. The effect of dietary control and carbohydrate supplementation on the immune and hormonal responses to rowing exercise. Appl Physiol Nutr Metab. 2006; 31:588–596.

17. Alvandi H, Salehzadeh K, Najafzade MR, Taheri Kalani A. The effect of strength training on anti-inflammatory cytokines, cortisol and testosterone in overweight men. Euro J Exp Bio. 2014; 4:296–302.
18. Alberti S, Cevenini E, Ostan R, Capri M, Salvioli S, Bucci L, Ginaldi L, De MM, Franceschi C, Monti D. Age-dependent modifications of Type 1 and Type 2 cytokines within virgin and memory CD4+ Tcellsinhumans. Mech Ageing Dev. 2006; 127:560–566.

19. Ogawa K, Oka J, Yamakawa J, Higuchi M. Habitual exercise did not affect the balance of type 1 and type 2 cytokines in elderly people. Mech Ageing Dev. 2003; 124:951–956.

20. Xiang L, Rehm KE, Marshall GD Jr. Effects of strenuous exercise on Th1/Th2 gene expression from human peripheral blood mononuclear cells of marathon participants. Mol Immunol. 2014; 60:129–134.

21. Schreiber GH, Schreiber RD. Interferon-gamma. In : Angus W, Thompson A, Lotze MT, editors. The Cytokine Handbook. 4th ed. Academic Press;2003. p. 567–601.
22. Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O'Shea JJ. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000; 164:1768–1774.

24. Prokopchuk O, Liu Y, Wang L, Wirth K, Schmidtbleicher D, Steinacker JM. Skeletal muscle IL-4, IL-4Ralpha, IL-13 and IL-13Ralpha1 expression and response to strength training. Exerc Immunol Rev. 2007; 13:67–75.
25. Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci. 2007; 120:670–681.

26. De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid Redox Signal. 2005; 7:1356–1366.

27. Suzuki K, Nakaji S, Kurakake S, Totsuka M, Sato K, Kuriyama T, Fujimoto H, Shibusawa K, Machida K, Sugawara K. Exhaustive exercise and type-1/type-2 cytokine balance with special focus on interleukin-12 p40/p70. Exerc Immunol Rev. 2003; 9:48–57.
28. Cannon JG. Inflammatory cytokines in nonpathological states. News Physiol Sci. 2000; 15:298–303.

30. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005; 98:1154–1162.

31. Hirose L, Nosaka K, Newton M, Laveder A, Kano M, Peake J, Suzuki K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev. 2004; 10:75–90.
32. Malm C, Sjodin TL, Sjoberg B, Lenkei R, Renstrom P, Lundberg IE, Ekblom B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol. 2004; 556:983–1000.

33. Toft AD, Jensen LB, Bruunsgaard H, Ibfelt T, Halkjaer-Kristensen J, Febbraio M, Pedersen BK. Cytokine response to eccentric exercise in young and elderly humans. Am J Physiol Cell Physiol. 2002; 283:C289–C295.
34. Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu Q, Kudoh S, Kowatari K, Nakaji S, Sugawara K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000; 81:281–287.

35. Huffman KM, Slentz CA, Bales CW, Houmard JA, Kraus WE. Relationships between adipose tissue and cytokine responses to a randomized controlled exercise training intervention. Metabolism. 2008; 57:577–583.

36. Chikanza IC, Grossman AB. Reciprocal interactions between the neuroendocrine and immune systems during inflammation. Rheum Dis Clin North Am. 2000; 26:693–711.

37. Saitoh M, Yanagawa T, Kondoh T, Miyakoda H, Kotake H, Mashiba H. Neurohumoral factor responses to mental (arithmetic) stress and dynamic exercise in normal subjects. Intern Med. 1995; 34:618–622.

38. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000; 52:595–638.
39. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003; 8:223–246.
40. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011; 10:319–329.

41. Izquierdo M, Ibanez J, Calbet JA, Navarro-Amezqueta I, Gonzalez-Izal M, Idoate F, Hakkinen K, Kraemer WJ, Palacios-Sarrasqueta M, Almar M, Gorostiaga EM. Cytokine and hormone responses to resistance training. Eur J Appl Physiol. 2009; 107:397–409.

42. Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise: cytokine kinetics. Exerc Immunol Rev. 2000; 8:6–48.
43. Ho CS, Lopez JA, Vuckovic S, Pyke CM, Hockey RL, Hart DN. Surgical and physical stress increases circulating blood dendritic cell counts independently of monocyte counts. Blood. 2001; 98:140–145.

44. Upham JW, Lundahl J, Liang H, Denburg JA, O'Byrne PM, Snider DP. Simplified quantitation of myeloid dendritic cells in peripheral blood using flow cytometry. Cytometry. 2000; 40:50–59.

45. Lancaster GI, Khan Q, Drysdale PT, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol (1985). 2005; 98:565–571.

46. Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997; 158:5589–5595.
47. Pedersen BK, Rohde T, Ostrowski K. Recovery of the immune system after exercise. Acta Physiol Scand. 1998; 162:325–332.

48. Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006; 1069:62–76.

49. Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. 2004; 10:91–106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download