Abstract
Evaluation and screening of vaccines against tuberculosis depends on development of proper cost effective disease models along with identification of different immune markers that can be used as surrogate endpoints of protection in preclinical and clinical studies. The objective of the present study was therefore evaluation of subcutaneous model of M.tuberculosis infection along with investigation of different immune biomarkers of tuberculosis infection in BALB/c mice. Groups of mice were infected subcutaneously with two different doses : high (2×106 CFU) and low doses (2×102 CFU) of M.tuberculosis and immune markers including humoral and cellular markers were evaluated 30 days post M.tuberculosis infections. Based on results, we found that high dose of subcutaneous infection produced chronic disease with significant (p<0.001) production of immune markers of infection like IFNγ, heat shock antigens (65, 71) and antibody titres against panel of M.tuberculosis antigens (ESAT-6, CFP-10, Ag85B, 45kDa, GroES, Hsp-16) all of which correlated with high bacterial burden in lungs and spleen. To conclude high dose of subcutaneous infection produces chronic TB infection in mice and can be used as convenient alternative to aerosol models in resource limited settings. Moreover assessment of immune markers namely mycobacterial antigens and antibodies can provide us valuable insights on modulation of immune response post infection. However further investigations along with optimization of study protocols are needed to justify the outcome of present study and establish such markers as surrogate endpoints of vaccine protection in preclinical and clinical studies in future.
Bacillus Calmette Guerin (BCG), only licensed Tuberculosis (TB) vaccine, has afforded limited protection against TB despite of its widespread use in developing countries like India (1,2). To overcome this limitation, a number of different vaccination strategies have been investigated using different animal models of TB infection (3,4). However, animal models that strictly reproduce the human host-M.tuberculosis (MTB) relationship are required to evaluate new vaccines and therapies (5). Aerosol model by far is most widely used route to establish TB infection in mouse since it replicates immunological events in human leading to slow progressive disease development. However, requirement of Biosafety level-3 (BSL-3) facilities and high maintenance cost limits their usage in many resource limited settings of developing world. Other routes of infection such as subcutaneous, intravenous and intranasal have been investigated in other studies for development of TB model in animals (6,7,8). Although subcutaneous route may not mimic actual development of infection in animals, however, it can be used as convenient alternative to aerosol route (9,10) in resource limited settings. Dose of MTB infection used is another contributing factor, since outcome of successful infection generally depends on amount of bacteria colonizing in lungs and other organs of host animal used (11). Thus along with route of infection, standardization of optimal dose required for infection is mandatory in development of proper disease model of TB infection.
Another major aspect of vaccine evaluation is identifcation of appropriate markers that can be used as surrogate endpoints of protection. Infection with MTB leads to diverse immune response, thereby producing wide range of biomarkers (11). Whether or not infection will lead to development of disease depends on the outcome of a complex interaction between the pathogen and the host's immune response (12). Therefore, based on our understanding of the pathogen-host interactions, the design of superior vaccines or drugs against mycobacterial infections can be facilitated. Assesment of biomarkers, especially MTB antigens and antibodies produced during infection provide us useful insight with respect to development of disease and may also increase our understanding about their production during infection. Moreover, with increase in ethical concerns regarding number of experimental animals used and sacrificed in vaccination studies, identification and evaluation of such biomarkers as surrogate endpoints are need in preclinical evaluation of such studies in future.
Keeping the existing questions in mind, the objective of the study was to evaluate subcutaneous model of TB using two different doses of MTB infection in BALB/c mice. Apart from evaluation of subcutaneous model, the study also focused on evaluation of different immune markers post MTB infection which can be used as surrogate endpoints for evaluation of different vaccine candidates under preclinical and clinical stage of development.
Female BALB/c mice, 6~8 weeks old, were obtained from National Institute of Nutrition (NIN, India), Hyderabad. Mice were housed under aseptic conditions and provided with food and sterile water. Prior to experiments, all mice were acclimatized for 15~20 days.
MTB H37Rv antigens Ag85B, ESAT-6, CFP-10, Gro-ES, and Hsp16 along with Monoclonal antibodies against Hsp16 (alpha-crystalline like-Rv2031c, hspX), Hsp65 (Rv0440, cpn60.2, GroEL) and Hsp71 (Rv0350, DnaK), were obtained from Colorado State University, USA under the TB research materials and vaccine testing contract (NO1-AI-75320). The secondary antibody rabbit anti-mouse IgG-HRP was obtained from Genei, Banglore, India. MTB purified protein derivative (PPD) was obtained from Span Diagnostics, Banglore, India.
The MTB H37Rv was grown in 7H9 Middlebrook Broth (Himedia laboratories, India) to mid log phase. The bacterial suspension was diluted in phosphate buffered saline (PBS) and adjusted according to the number 1 McFarland scale. Depending upon the load of mycobacteria to be infected, the cultures were serially diluted in sterile saline. Mice were divided into two different experimental groups (n=10, each group), and infected subcutaneously with 2×106 CFU (for high dose), and 2×102 (low dose). All procedures of culturing and infection were carried out in BSL facilities. A control group of mice (n=10) were separately maintained and sham immunized with sterile saline. 30 days after MTB infection, blood was collected to obtain serum and used for immunological marker analysis.
MTB heat shock proteins (Hsps) 16, 65, 71 were detected using in house developed ELISA method as described elsewhere (13). Total IgG was estimated using ELISA protocol as described earlier by Husain et al. (2). The antibody response against MTB antigens (Ag85, ESAT-6, CFP-10, Gro-ES, and Hsp16) were evaluated using in house ELISA method developed by Kashyap et al. Briefly, the 96-well microtiter plates (MaxisorpImmunoplate, Nalge Nunc International, Naperville, IL, USA) were coated with MTB antigens. After 3 hours of incubation at 37℃, the plates were washed and blocked with 0.25% bovine serum albumin (BSA) in PBS pH 7.4. After 2 hours of incubation plates were washed twice and kept overnight at 4℃. Next day plates were incubated with serum samples (1:400 diluted) in PBS. After 30 minutes of incubation plates were washed and incubated with rabbit anti-mouse IgG, HRP conjugate (1:10,000) for 30 min. For colour development tetramethyl benzidine in hydrogen peroxide (TMB/H2O2) substrate was added and incubated for 10 min. The reaction was terminated by adding 2.5 N sulphuric acid and the absorbance of colour in each well was read at 450 nm. Anti-PPD titre was estimated by protocol for antibody detection described earlier.
Thirty days after MTB infection, mice from respective groups (n=7) were sacrificed. Lungs and spleen were isolated, homogenized and serially diluted. These serially diluted homogenates were innoculated in Middle brook 7H9 liquid medium along with oleic acid, albumin, dextrose and catalase (OADC) enrichment and antibiotic supplements in BacT culture bottles (Biomeriux, France) and were incubated at 37℃ in BacT/Alert system (Biomerieux) for 28~35 days. Mycobacterial load in organs was determined in terms of mean time taken by organ cultures from respective groups to become positive in BacT/Alert system as described elsewhere by Kolibab et al. (14).
Cytokine Interferon gamma (IFN-γ) was assessed in spleenocytes as per manufacturer's instruction (Bender Med System, Austria). In brief, anti-IFN-γ-monoclonal coating antibody was adsorbed onto micro wells. After two hours of incubation at room temperature, the wells were washed and blocked with 0.5% BSA in PBS. After one hour, spleenocytes (1:400 diluted) followed by biotin-conjugated anti-cytokine antibody was added to the coated wells. After another two hours of incubation, streptavidin-HRP (horseradish peroxidase) was added to the wells. After one hour of incubation, substrate solution reactive with HRP was added to the wells. The reaction was terminated by the addition of 2.5 N sulphuric acid and the absorbance of colour was read at 450 nm.
A section was prepared from the base of the apical lobe and from the diaphragmatic lobe of the left lung, representing two distinct regions of the organ. Duplicate sections were stained with haematoxylin and eosin and Van Gieson in order to aid visualization of fibrous tissue. The two sections were scored in a blinded fashion for the following features: percentage of the section occupied by granulomatous inflammation, healthy lung, interstitial pneumonitis, and necrosis. The presence of epithelioid cells and the extent of fibrosis and lymphocytic infiltration (and whether around vessels or in granulomas in both cases) were also assessed. Amount of granulomatous lesions were scored in terms of percentage (%) occupied by them in lungs.
All protocols for animal experiments were approved by Institutional Animal Ethics Committee of Central India Institute of Medical Sciences, Nagpur.
Data are expressed as mean±standard deviation (SD). Graphs were plotted using Graph Pad Prism 6 software. For multiple comparisons Paired t-test was used for obtaining statistical significance in different dose groups. p<0.05 was considered statistically significant (**) and p<0.001 for highly significant (***) values.
Antibodies were estimated 30 days post MTB infection. Blood was harvested from different groups of mice to obtain serum. Fig. 1A and B indicates total IgG and anti-PPD titres in different dose and control groups. Mice infected with high dose showed significantly (p<0.001) high total IgG and anti-PPD response comapred to low dose and control group. Antibody titres against panel of MTB H37Rv antigens (ESAT-6, CFP-10, GroES, Hsp-16, 45kD, Ag85B) showed similar results with significantly higher titres against Ag85B, ESAT-6, and CFP-10 in high dose group (Fig. 2). On the contrary, levels of antibodies against GroES, 45kDa and Hsp16 were found to be similar in both dose groups.
MTB Hsp (s) were analyzed in mice samples to determine course of antigen response in different groups of mice post infection. Fig. 3 shows mean (a) Hsp 16 (b) Hsp 65 and (c) Hsp71 antigen response in different dose and control group. Mice infected with high dose of MTB showed robust Hsp 65 and 71 levels significant (p<0.001) compared to low dose groups, while both dose groups showed similar levels of Hsp 16 post TB infection.
Mice from respective groups (n=7) were sacrificed after 30 days of MTB infection and mycobacterial load was determined by inoculating serially diluted lung and spleen homogenates in Middle brook 7H9 liquid medium along with OADC enrichment and antibiotic supplements in BAC/T culture bottles (Biomeriux) and incubated at 37℃ in BacT/Alert system (Biomerieux) for 28 days. Mean time required for growth in BacT was taken as a correlate of mycobacterial load in respective organs of mice group. High dose group showed comparatively higher bacterial burden in lungs and spleen which was significant (p<0.001) compared to low dose group and control group (Fig. 4). Mean positive time for high dose group in lungs and spleen was around 18.5±2.0, 19±0.2 day's compared to 28±1.5, 32±0.5 days taken by low dose group. No bacterial burden was observed in sham infected control group.
Fig. 5A shows photomicrograph of histopathological examination of lung tissue of mice infected with different dose groups. Thirty days after MTB infection, mice from respective groups were sacrificed and organs (lungs and spleen) were isolated. Tissues were fixed in 10% formalin, paraffin sectioned and stained with Hematoxylin and Eosin. Mice infected with high dose of MTB infection induced chronic lung pathology with around 70% lession compared to 40% observed in low dose group. In terms of infiltration, we observed similar results with high infiltration rate in lungs of mice infected with high dose (p<0.001) compared to low dose group (Fig. 5B). For interferon analysis, isolated spleen cells were homogenized and stimulated with PPD. High dose group showed significantly higher levels of IFN-γ (p<0.001) post infection compared to low dose group and control (Fig. 5C) These levels correlated well with chronic lung pathology and higher bacterial burden in lungs of mice thereby indicating severity of infection.
Designing and screening of better vaccine candidates depends on development of proper diseased models of infection (15). Aerosols models by far have been used as gold standard for development of TB infection in mice and are used for over a decade. Although aerosol route of infection is undoubtedly best route in terms of mimicking the actual mechanism of infection, it generally requires elaborate laboratory facilities and therefore cannot be used in resource limited settings. Thus, alternate models to aerosol routes need to be investigated for laboratories aiming to evaluate vaccine efficacy of new molecules under evaluation. Subcutaneous and intravenous routes were popular for infecting the animals prior to discovery of aerosol inhalation chamber (11). The limitation associated with former procedures is the amount of dose required for the infection. Since, the amount injected and amount reaching the organ may vary due to various immunological barriers of host immune system.
In the present study, we therfore investigated subcutaneous model for development of TB using two different doses of MTB infection in mice. Rationale for selection of different doses was to optimize best dose for development of MTB infection in mice. Groups of mice were challenged subcutaneously with high and low dose of MTB. Various correlates of antimycobacterial immunity were studied in both dose groups at five weeks post MTB infection. Based on the results, we found that mice infected with high dose of subcutaneous infection developed chronic disease with robust IFN-γ levels in splenocytes and high titers of MTB antigens and antibodies in serum as compared to low dose group. Histopathological investigations revealed chronic lung pathology in high dose group with around 70% lesion and high lymphocytic infiltration rate which correlated well with high load of bacterial burden in lungs. On the other hand, low dose group show moderate level of infection and comparably low levels of cytokine, organ bacterial load and anti mycobacterial antibodies.
The present study thus provides some useful insights in terms of evaluating efficacy of subcutaneous route and assessment of role of multiple immune markers post MTB infection. From the obtained results, it becomes evident that antigen and antibodies plays a pleomorphic role in TB infection comparable to already established T cell response. Various antigens and antibodies produced during disease states have long been used for diagnosis of TB infection in humans (1). MTB H37Rv antigens like Ag85B, ESAT-6, CFP-10, and Hsp-16 are regarded as major immunodominant antigens produced after establishment of TB infection (16). Although cytokines such as IFN-γ are key mediators of immunity after infection, during the past few years, there are lots of published reports which suggest a key role of humoral response in TB immunology. In recent studies, it has been shown that antibody responses are essential to contain mycobacterial infection, and there is a synergy and mutual interdependence between cell mediated and humoral immunity (17). Zuniga et al., in their review article have discussed briefly about different cellular and humoral mechanisms evolved in control of TB infection and also suggested a possible role of B cells in modulating immune response in TB (18). Report by Kozakiewicz et al. suggests B cell plays a vital role in shaping immune response against MTB infection (19). Our results thus are in agreement with reported evidences supporting the role of antibodies and antigens in immune response after TB infection. Assessment of multiple biomarkers including markers of humoral immunity may thus provide valuable evidence regarding development of TB infection and can be used as surrogate end points for screening of vaccine efficacy in various candidate molecules.
Another major observation of the study is the correlation of all the TB markers with lung pathology and MTB load in organs of the mice. Each year, large number of animals are utilized for studying and evaluating vaccines for TB and other infectious diseases. Although there is no exact figure, it is estimated that vaccine research, development, production, and quality control constitutes around 15% of the total number of animals used in biomedical research (20). These animals are used at various stages including screening to adjuvant selection, immunogenicity & safety studies, tests for route & dose of administration along with formulation etc. Also large number of animals are sacrifice in order to study bacterial burden in organ and for histopathological procedures. Thus evaluation of markers that can be used as an alternatives to such procedures are needed to minimize the number of animal sacrifice thereby reducing ethical constrains on use of animal for vaccination studies. In the present study, we estimated biomarkers of humoral and cellular immunity and found that high titres of such biomarkers significantly correlated with bacterial load and lung pathology. Our results thereby support our mentioned hypothesis suggesting that evaluated markers can be used as alternative to CFU studies and histopathological procedures) especially in screening and standardization assays in mice model. However these are just preliminary observations, and require extensive further investigation in different animal models using same route of infection.
There are few limitations associated with study. Major limitation was our inability to use other routes of infection such as Intravenous for comparison of disease development and biomarker outcome with subcutaneous route. Another limitation was lack of evaluation of markers at different time points after MTB infection.
To conclude, high dose of subcutaneous infection produces chronic TB infection in mice and can be use as convenient alternative to aerosol models in resource limited settings. Moreover, assessment of immune markers such as MTB antigens and antibodies post MTB infection provides valuable insights on modulation of immune response post infection. However further investigations along with optimization of study protocols are needed to justify the present study outcome and establish such markers as surrogate endpoints of vaccine protection in preclinical and clinical studies in future.
Figures and Tables
 | Figure 1(A) Mean Total IgG and (B) anti-PPD levels in serum of mice (n=7, each group) in high dose, low dose and control groups. Antibody levels were estimated 30 days after development of TB infection. Paired t-test was used to compare and obtain statistical significance. Error bars indicate standard error of mean. **Represents significant (p<0.05) and ***represents highly significant (p<0.001) values. |
 | Figure 2Scatter plot of antibody levels against panel of MTB H37Rv antigens (a) Ag85B (b) 45kDa (c) Hsp 16 (d) GroEs (e) ESAT-6 and (f) CFP-10 in serum of mice (n=7, each group) after TB infection. Paired t-test was used to compare and obtain statistical significance. Error bars indicate standard error of mean. **Represent significant (p<0.05) and ***represents highly significant (p<0.001) values. |
 | Figure 3Mean mycobacterial Hsp levels (a) mHsp 16 (b) mHsp-65 and (c) mHsp-71 in serum of mice (n=7, each groups) in different dose groups. Error bars indicate standard error of mean. **Represents significant (p<0.05) and ***represents highly significant (p<0.001) values. |
 | Figure 4Shows mean growth time of MTB in lung and spleen homogenates of mice (n=7) collected 30 post MTB infection in different dose group. Mice from respective groups were sacrificed and mycobacterial load was determined by inoculating serially diluted lung homogenates in Middle brook 7H9 liquid medium and incubating at 37℃ in BacT/Alert system (Biomerieux) for 30 days. Bars represent mean time required for growth in BacT was taken as a correlate of load in respective organs of mice group. Data are shown as ±SD. **Represent significant (p<0.05) values. |
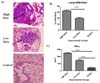 | Figure 5(A) Representative Histopathology of lungs section of mice (high dose, low dose and control) stained with hematoxylin and Eosin. (B) along with percentage lymphocytic infiltarion in different dose groups and control. Mice were sacrificed 30 days after MTB infection. Arrows indicate percentage of lesions covered in lungs. (C) Shows mean INF-γ levels in lung homogenates of mice at different dose groups. Errors bars indicate standard error of mean. **Represent significant (p<0.05) and ***represents highly significant (p<0.001) values. |
ACKNOWLEDGEMENTS
AAH would like to thanks Indian Council of Medical Research (ICMR), Govt. of India, for Award of senior research Fellowship. All Authors would like to thank Central India Institute of Medical Sciences for funding this study. Authors also acknowledge Colorado State University, USA, for providing MTB antigens and antibodies under the TB Research Materials and Vaccine Testing Contract (NO1-AI-75320). Authors also thank Ms Seema Shekhawat for her assistance in manuscript corrections.
References
1. Kashyap RS, Nayak AR, Husain AA, Gaherwar HM, Purohit HJ, Taori GM. Tuberculosis in India: the continuing challenge. Curr Sci. 2013; 105:597–606.
2. Husain AA, Kashyap RS, Kalorey DR, Warke SR, Purohit HJ, Taori GM, Daginawal HF. Effect of repeat dose of BCG vaccination on humoral response in mice model. Indian J Exp Biol. 2011; 49:7–10.
3. Parida SK, Kaufmann SH. Novel tuberculosis vaccines on the horizon. Curr Opin Immunol. 2010; 22:374–384.

4. Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008; 372:164–175.

5. Morais Fonseca D, Rosada RS, e Paula MO, Wowk PF, Franco LH, Soares EG, Silva CL, Deperon Bonato VL. Experimental tuberculosis: designing a better model to test vaccines against tuberculosis. Tuberculosis (Edinb). 2010; 90:135–142.

6. Sugawara I, Udagawa T, Aoki T, Mizuno S. Establishment of a guinea pig model of latent tuberculosis with GFP-introduced Mycobacterium tuberculosis. Tohoku J Exp Med. 2009; 219:257–262.

7. Srivastava S, Ayyagari A, Dhole TN, Krishnani N, Nyati KK, Dwivedi SK. Progression of chronic pulmonary tuberculosis in mice intravenously infected with ethambutol resistant Mycobacterium tuberculosis. Indian J Med Microbiol. 2008; 26:342–348.

8. Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004; 114:1790–1799.

9. Bonanni D, Rindi L, Lari N, Garzelli C. Immunogenicity of mycobacterial PPE44 (Rv2770c) in Mycobacterium bovis BCG-infected mice. J Med Microbiol. 2005; 54:443–448.

10. Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol. 2010; 17:1170–1182.

11. Gupta UD, Katoch VM. Animal models of tuberculosis for vaccine development. Indian J Med Res. 2009; 129:11–18.
12. Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb). 2004; 84:93–101.

13. Shekhawat SD, Jain RK, Gaherwar HM, Purohit HJ, Taori GM, Daginawala HF, Kashyap RS. Heat shock proteins: possible biomarkers in pulmonary and extrapulmonary tuberculosis. Hum Immunol. 2014; 75:151–158.

14. Kolibab K, Yang A, Parra M, Derrick SC, Morris SL. Time to detection of Mycobacterium tuberculosis using the MGIT 320 system correlates with colony counting in preclinical testing of new vaccines. Clin Vaccine Immunol. 2014; 21:453–455.

15. Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go. PLoS Pathog. 2012; 8:e1002607.

16. Kashyap RS, Nayak AR, Gaikwad S, Jain RK, Daginawala HF, Taori GM. Biomarkers in pulmonary and extra pulmonary tuberculosis. In : Proceedings of theInternational conference on Advances in developing Affordable Invitro molecular Diagnostics; 2013. p. 73–86.
17. Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009; 157:235–243.

18. Zuniga J, Torres-García D, Santos-Mendoza T, Rodriguez-Reyna TS, Granados J, Yunis EJ. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Dev Immunol. 2012; 2012:193923.
19. Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, Flynn JL, Porcelli SA, Jacobs WR Jr, Chan J. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013; 9:e1003472.

20. Acosta A, Norazmi MN, Hernandez-Pando R, Alvarez N, Borrero R, Infante JF, Sarmiento ME. The importance of animal models in tuberculosis vaccine development. Malays J Med Sci. 2011; 18:5–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download