INTRODUCTION
Skin cancer, the most common malignancy worldwide, is classified into two main categories, non-melanoma skin cancer (NMSC) and melanoma (1). Basal cell cancer (80%) and squamous cell cancer (16%) are the major subtypes of NMSC (1). Although NMSC is the most common skin cancer, it is rarely lethal (2). Melanoma, which originates from melanocytes, accounts for <4% of all skin cancer cases but is the major cause of death from skin cancer (3). Melanocytes are melanin-producing cells found mainly in the skin and eyes. In the skin, melanocytes reside in the bottom layer of the epidermis. Exposure to ultraviolet (UV) radiation increases melanin production by melanocytes. The biological function of melanogenesis is to protect the tissues under the skin from DNA-damaging UV exposure (3). Although the causes of melanoma are unknown, DNA damage because of UV exposure and genetic predisposition may play a role (4,5,6). It is estimated that approximately 76,100 patients in USA will be diagnosed with melanoma in 2014 and 9,710 will die of malignant melanoma (7). While the incidence rates of most cancer types are declining, the incidence of melanoma is gradually increasing (7). Although localized melanoma may be curable by surgical resection, patients with distant metastases have an extremely poor prognosis with an estimated 5-year survival rate of <16% (7). In this review, we summarize the currently available drugs and therapies of malignant melanoma and suggest the possible use of adoptive cell therapy (ACT) with cytokine-induced killer (CIK) cells as an additional therapeutic approach for malignant melanoma.
CURRENT TREATMENT OF MALIGNANT MELANOMA
Before 2011, there were only two agents approved by the US Food and Drug Administration (FDA) for the treatment of metastatic melanoma: the chemotherapeutic agent dacarbazine (an alkylating compound) and interleukin-2 (IL-2) (8). However, both drugs show a low response rate (5%~20%) and have considerable adverse effects (9). Over the last decade, a better understanding of genetic and molecular pathogenesis of melanoma, in particular mutations that increase the risk of this cancer, has led to new treatment approaches. Hyper-activation of mitogen-activated protein kinase (MAPK) signaling (namely, the RAS-RAF-MEK-ERK pathway), is the main characteristic of malignant melanoma detected in up to 75% of human melanomas. The most common mutations in human melanomas are found in neuroblastoma RAS viral oncogene homolog (NRAS, 15~25%) and v-raf murine sarcoma viral oncogene homolog B (BRAF, 50~70%) (10,11). FDA has approved several selective inhibitors of BRAF (vemurafenib and dabrafenib) and MEK (trametinib). Vemurafenib and dabrafenib target V600E-mutated BRAF, which is the most common BRAF mutation (approximately 90%) (9). All chemical inhibitors improve progression-free survival and overall survival of metastatic melanoma patients with the BRAF V600E or V600K mutation (12,13). However, targeted therapy of malignant melanoma is often limited by the emergence of acquired drug resistance 6~7 months after administration (14). Drug resistance occurs via reactivation of the MAPK pathway or activation of an alternative signaling pathway such as PI3K-AKT-mTOR (13,15). As a result, most patients relapse with lethal drug resistance.
In addition, FDA also have approved monoclonal antibody (mAb) therapeutics, such as ipilimumab targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) receptor and pembrolizumab targeting programmed death 1 (PD-1) receptor. The purpose of immune checkpoint blockade with anti-CTLA4 and anti-PD-1 mAb is to restore the immune response. CTLA-4 and CD28 share B7 ligands, for example B7-1 (CD80) and B7-2 (CD86). CD28 provides the second signal required for T-cell activation after the first signal of antigen recognition through T cell receptor (TCR), whereas CTLA-4 inhibits T cell activation (16,17). Although the mechanisms of CTLA-4 action are still not fully understood, CTLA-4 may inhibit T-cell activation by competing with B7 ligand binding to CD28 or by interfering with CD28 signaling. Thus, CTLA-4 blockade with mAb can restore antitumor T cell immunity (18). A recent study shows that ipilimumab can bind CTLA-4 expressed on melanoma cells, which induce antibody-dependent cell-mediated cytotoxicity by natural killer (NK) cells and γδT lymphocytes (19). Another immune checkpoint molecule is PD-1, a T-cell co-inhibitory molecule. PD-1 is expressed on activated T cells and negatively regulates T-cell response (16,20,21,22). Most melanoma and solid tumor cells express PD-1 ligand (PD-L1) on their surfaces (23). PD-1 blockade can enhance the antitumor activity of T cells by inhibiting the PD-1-PD-L1 interaction (24). Pembrolizumab (anti-PD-1 mAb) was approved by the FDA on September 4, 2014. Although immune checkpoint blockades have more durable responses than MAPK inhibitors, grade 3 or 4 immune-related adverse events were observed in ~15~25% of patients; these events included dermatitis, colitis, hypophysitis, hepatitis, pneumonitis, and neuromuscular toxicity (25,26,27,28). In addition, clinical responses to immune checkpoint blockades are generally slow (18).
ADOPTIVE CELL THERAPY OF MELANOMA WITH TUMOR-INFILTRATING LYMPHOCYTES
Adoptive cell therapy (ACT) is a form of cancer immunotherapy for malignant melanoma, which entails the transfer of immune cells such as tumor-infiltrating lymphocytes (TILs), genetically modified T cells, or NK cells. After host lymphodepletion, adoptive transfer of TILs increases the objective response rate by >51% and durable response more than conventional chemotherapy and targeted therapy do (29). Lymphodepletion prior to ACT has various advantages, including the elimination of Treg or myeloid-derived suppressor cells and increase of the cytokine levels in the serum (30,31,32). So far, TIL therapy has been the most effective ACT treatment for patients with metastatic melanoma. However, it is difficult to obtain tumor tissue to isolate TILs, and TILs from many patients fail to expand sufficiently in culture. Therefore, TIL therapy has been applied to only a limited number of patients (33). To increase the clinical application of TILs, genetically modified T cells are being developed. T lymphocytes expressing αβTCRs recognize tumor-associated antigens on the surface of MHC molecules on tumor cells. Some tumor-associated antigens have been identified, including melanoma antigen recognized by T cells 1 (MART-1) and cancer testis antigen (NY-ESO-1) (34,35). T cells expressing antigen-specific TCR can be made by genetic modification by using viral or non-viral transduction of peripheral blood lymphocytes (PBLs). Although genetic modification might increase the clinical application of T cell therapy, objective responses (19~30% of patients) were still lower than with autologous TILs (36,37). In addition, MART-1 and gp100-specific TCRs cause on-target toxicity in approximately half of patients, which leads to destruction of normal melanocytes in the skin (rash), eye (uveitis), and/or ear (hearing loss) (37). This suggests that safer and more effective antigens are needed. In addition, the mechanisms of action restricted to MHC I are a limitation for antigen-specific T cell therapy for MHC I-negative tumor cells.
ADOPTIVE CELL THERAPY OF MELANOMA WITH NATURAL KILLER CELLS
NK cells are a type of cytotoxic lymphocytes that function in innate immunity. They can lyse tumor cells without prior immunization or MHC-restriction (38). NK cell-mediated tumor cell killing depends on the balance between inhibitory and activating receptors and their signaling. The inhibitory receptors, which bind to MHC-I molecules, are present on most normal cells and prevent their killing by NK cells. Tumor cells often decrease the expression of MHC molecules, which allows them to avoid T cell-mediated killing (39,40) but makes such tumor cells potential targets of NK cells. However, tumor cells expressing MHC-I molecules suppress autologous NK cell-mediated killing (41). For this reason, haploidentical allogeneic NK cells have been successfully used for cancer immunotherapy without causing graft-versus-host disease (42). Although allogeneic NK cells eventually lead to immune-mediated rejection by host immunity, NK cell-based therapy has shown promising results in hematological cancer patients (42). However, in a clinical trial with melanoma patients, although a high level of circulating autologous NK cells persisted for several months, they failed to mediate tumor regression (43).
ADOPTIVE CELL THERAPY OF MELANOMA WITH CYTOKINE-INDUCED KILLER CELLS
Cytokine-induced killer cells are ex vivo-activated lymphocytes generated by culturing peripheral blood mononuclear cells (PBMCs) with the timed addition of IFN-γ, anti-CD3 antibodies, and IL-2 for >2 weeks (44). Anti-CD3 antibodies and IL-2 are essential for proliferation and activation of CIK cells (45). Heterogeneous CIK cells consist of two major populations, CD3+CD56- and CD3+CD56+ cells, and a relatively minor fraction of CD3-CD56+ cells. The CD3+CD56+ cells are the most potent cytotoxic cells. Interestingly, during ex vivo expansion, they originate from CD3+CD8+CD56- cells but not from CD3+CD56+ cells (46). Cytotoxicity of CIK cells is mainly mediated by perforin (47) and depends on the activating receptors such as NKG2D (48), NKp30, and DNAM-1 (49). CIK cells have the following advantages for cancer immunotherapy in comparison with other ACT approaches: 1) they can be easily generated and produced in large quantities by expansion from PBMCs ex vivo (50); 2) they exhibit non-MHC-restricted cytotoxic activity (49); and 3) they are effective in eliminating multidrug-resistant tumor cell lines (51,52,53,54). In addition, the antitumor activity of CIK cells can be increased by combination therapy without an increase in adverse effects and by co-culturing them with tumor lysate-pulsed dendritic cells (55,56).
In the last decade, many preclinical studies have demonstrated the antitumor activity of CIK cells against various tumor cells such as hepatoma (57), leukemia (58), as well as lung (59), ovarian (60), renal (61)and gastric (62) cancers. However, only a few studies have been reported on melanoma. Gammaitoni and colleagues reported that patient-derived CIK cells are able to kill not only autologous metastatic melanoma cells in vitro and in an in vivo xenograft mouse model, but also putative melanoma cancer stem cells (63). Such cells, which have stemness characteristics, are well known to cause relapse and drug resistance (64). This implies that CIK-cell therapy may be effective for treatment of patients with metastatic melanoma and also for prevention of relapse and metastasis.
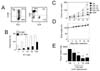
Here, we provide additional evidence that CIK-cell therapy can effectively eliminate melanoma in an in vivo xenograft mouse model. CIK cells were generated from PBMCs of healthy volunteers. PBMCs were isolated by Ficoll-Hypaque density centrifugation, washed three times with PBS and cultured in the presence of immobilized anti-CD3 antibody (5 µg/ml) and recombinant human IL-2 (700 U/ml) for 5 days. The cell suspension was further incubated in complete medium containing IL-2 only (170 U/ml) for 9 days. IL-2 and medium were replenished every 2 or 3 days (60). Cell density was maintained at ~1×106 cells/ml. On day 14, the viability of expanded cell populations was 85~90%. Cell phenotypes were examined by flow cytometry. CIK cell populations contained 93% CD3+, 5% CD3-CD56+, 47% CD3+CD56+, 11% CD4+, and 74% CD8+ cells (Fig. 1A), which was typical for a heterogeneous CIK cell population.
To determine the anti-tumor activity of CIK cells, two cell lines were used. LOX-IMVI cell lines are human malignant melanoma and have been used widely utilized for drug screening and molecular target identification (65,66,67). K-562 cell lines are human leukemic cells and used as reference target cells. Both cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and were grown in the RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin [20]. A 4-h 51Cr release assay revealed that CIK cells destroyed 11%, 16%, and 38% of LOX-IMVI cells at the effector:target ratios of 10:1, 30:1 and 100:1, respectively (Fig. 1B). Strong CIK cell cytotoxicity was also observed against K562 cells.
The nude mouse xenograft assay was used to examine the in vivo antitumor activity of CIK cells. LOX-IMVI cells (1.5×106) in 300 µl of PBS were injected subcutaneously on day 0, followed by intravenous once-a-week injection of CIK cells (see below for doses) and adriamycin (used as a reference drug; 2 mg/kg). On day 15, mice were sacrificed and the tumor mass and body weight (to determine toxicity) were measured. In control mice, LOX-IMVI cells grew to a tumor volume of 254±43 mm3 (n=7) (Fig. 1C). A strong anti-tumor effect of CIK cells was observed (Fig. 1E). CIK cells injected at doses of 1×106, 3×106, and 10×106 cells per mouse inhibited tumor growth by 34%, 57%, and 76%, respectively. Adriamycin also strongly inhibited the growth of LOX-IMVI cells (Fig. 1C). In control mice, the weight of LOX-IMVI cells reached 1,676±530 mg at 15 days after implantation. CIK cells injected at doses of 3×106 and 10×106 cells per mouse reduced tumor weight by 52% and 76%, respectively. Adriamycin also strongly inhibited tumor growth. CIK cells did not affect the body weight gain of nude mice, indicating that CIK cells are not toxic (Fig. 1D). Thus, our preclinical efficacy data show that CIK cells can destroy LOX-IMVI cells in vitro and in vivo, and suggest that CIK cells might be a good candidate immunotherapy of melanoma.
CONCLUSION
Patients with metastatic melanoma have a high risk of metastases leading to death. So far, conventional chemo- and targeted therapies have been the first-line treatments, but they are often limited by drug resistance. Although combination therapy delays drug resistance, an increase in side effects needs to be carefully managed or treatments need to be discontinued. Over the last few years, CIK cell therapy has been evaluated in many clinical studies in patients with hepatocellular carcinoma, renal cell carcinoma, non-small cell lung cancer, and gastric cancer (68). CIK cell therapy improved the prognosis of these cancer patients (increased overall survival and progression-free survival) without significant adverse events. In most cancer patients, combination of CIK cell therapy with conventional treatment options shows better clinical outcomes than standard therapy alone. Unfortunately, there are no reports of clinical studies of the use of CIK cells for melanoma treatment. Preclinical data obtained by us and others, which demonstrate that CIK cells have sufficient antitumor activity against melanoma in vitro and in in vivo animal models, warrant clinical studies on adoptive cell therapy of melanoma with CIK cells.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download