Abstract
It is well established that TGF-β1 and retinoic acid (RA) cause IgA isotype switching in mice. We recently found that lactoferrin (LF) also has an activity of IgA isotype switching in spleen B cells. The present study explored the effect of LF on the Ig production by mouse peritoneal B cells. LF, like TGF-β1, substantially increased IgA production in peritoneal B1 cells but little in peritoneal B2 cells. In contrast, LF increased IgG2b production in peritoneal B2 cells much more strongly than in peritoneal B1 cells. LF in combination with RA further enhanced the IgA production and, interestingly, this enhancement was restricted to IgA isotype and B1 cells. Similarly, the combination of the two molecules also led to expression of gut homing molecules α4β7 and CCR9 on peritoneal B1 cells, but not on peritoneal B2 cells. Thus, these results indicate that LF and RA can contribute to gut IgA response through stimulating IgA isotype switching and expression of gut-homing molecules in peritoneal B1 cells.
B1 and B2 cells are the main two murine B cell lineages (1), the latter is named because they develop later in ontogeny than B1 cells. Mammalian splenic B2 cells are divided into marginal zone (MZ) and follicular (FO) B cells based on their phenotypes, micro-anatomical localization, and functions (2,3,4). B1 cells are the main B cell population in the peritoneal cavity in mice (5,6). B2/FO B cells, which are known as conventional B cells, primarily express monoreactive BCRs and require T cell help, whereas B1/MZ B cells, also called innate-like B cells, express polyreactive BCRs that bind multiple microbial molecular patterns and mainly mount early immune response via T cell-independent pathway (7,8,9,10,11).
To protect mucosal surface against pathogens, numerous IgA secreting B cells are located in the intestinal lamina propria (LP). The development of IgA B cells in the gut has been extensively studied, and it is generally accepted that these IgA B cells are primarily generated from B2 cells in organized follicular structures such as Peyer's patches (PPs) (12,13,14). However, it has become clear that peritoneal B1 cells contribute to reinforcement of the gut barrier by producing poly-specific IgA and therefore function as a first layer of immune defense at the gut mucosa (15). Several lines of evidence reveal that half of the IgA plasma cells in the LP are derived from peritoneal B1 cells (16,17,18). It is well established that TGF-β1 and retinoic acid (RA) induces IgA isotype switching in B cells (19,20,21,22,23,24). Further, these cytokines induce differential IgA isotype switching among peritoneal and splenic B cell subsets, in which peritoneal B1 and splenic MZ B cells switch more readily to IgA than peritoneal B2 and splenic FO B cells (25,26).
Lymphocyte subsets express unique patterns of homing molecules, and the various types of vascular endothelium in different tissues express their specific ligands, enabling migrating lymphocytes to reach their target tissue via site-specific pathways (27). Gut-tropic B cells express two homing-molecules on their surface, α4β7 and CCR9, which are associated with the mucosal trafficking of these cells. GALT-DC-derived RA is a key factor for imprinting gut-homing specificity on B cell through induction of α4β7 and CCR9 (28). Recently, we have demonstrated that RA is much more potent in the presence of TGF-β1 than on its own in inducing the expression of gut-homing molecules (24).
Lactoferrin (LF) is a highly cationic monomeric glycoprotein that is abundant in many mucosal surfaces and milk (29). LF is regarded as an important immunomodulator in innate and adaptive immunity, bridging the two immune responses (30). We have recently demonstrated that LF causes IgA and IgG2b isotype switching in spleen B cells (31). Nonetheless, it has not yet been defined whether LF can affect differentiation of peritoneal B cells.
In this study, we investigated the effect of LF on IgA production by peritoneal B cell subsets. We found that LF significantly induce peritoneal B1 cells to produce IgA isotype but not peritoneal B2 cells. This increase in IgA production was further augmented by RA. Moreover, the combination of LF and RA markedly enhanced the expression of the gut-homing molecules CCR9 and α4β7.
C57BL/6 mice were purchased and maintained in an animal environmental control chamber from Daehan biolink (Seoul, Korea). Animals were fed Purina Laboratory Rodent Chow 5001 ad libitum. Mice that were twelve to thirty-two weeks of age were used in this study. Animal care was performed in accordance with the institutional guidelines set forth by Kangwon National University.
RA and LPS (Escherichia coli O111:B4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bovine LF was supplied by Morinaga Milk Co., Ltd (Zama, Japan). Recombinant human TGF-β1 was purchased from R&D systems (Minneapolis, USA). The antibodies used in ELISA were purchased from Southern Biotechnology (Birmingham, USA).
To prepare murine peritoneal B cell suspension, peritoneum was flushed three times with 5 ml of PBS containing 2% FBS, the washout collected was centrifuged. The pellet was washed twice with HBSS and suspended in RPMI 1640 medium (Sigma) supplemented with 10% FBS, 50 µM 2-ME, 5 mM HEPES, penicillin (100 U/ml)/streptomycin (100 µg/ml). Since peritoneum primarily consists of macrophages and B cells, macrophages were depleted by incubation at 37℃ for 3 h using their adherent property in culture media. To separate peritoneal B1 and B2 cells, macrophage-depleted peritoneal cells were placed on Thy1.2- and CD43-dependent magnetic separation process sequentially (Miltenyi Biotech, Auburn, USA), in which CD43+ population contains B1 cells and CD43- population, B2 cells. This separation procedure resulted in >93% for B1 cells and >91% for B2 cells among B220+ cell population. A total of 5×105 cells/well were cultured in flat-bottomed, 48-well tissue culture plates (SPL, Pocheon, Korea) in a volume of 500 µl complete medium with added stimulants. A total of 2×105 cells/well were cultured in flat-bottomed, 96-well tissue culture plates (SPL) in a volume of 200 µl complete medium with added stimulants.
ELISAs were performed as described previously (32). Absorbance of reaction products was measured at 415 nm with an ELISA reader (VERSAMAX reader, Molecular Devices, Sunnyvale, USA).
Cells were stained with anti-mouse IgM-FITC (Southern-Biotech), anti-mouse CD43-biotin (clone L11; Miltenyi Biotech), anti-mouse IgA-FITC (SouthernBiotech), anti-mouse CD23-biotin (clone B3B4; Miltenyi Biotech), anti-mouse IgM-PE (SouthernBiotech) anti-mouse CD45R/B220-biotin (clone RA3-6B2; BD Pharmingen, San Diego, USA), anti-mouse CCR9-PE (clone 242503; R&D Systems), anti-mouse LPAM1 (α4β7)-biotin (clone DATK32; SouthernBiotech), and streptoavidin-allophycocyanin (eBioscience, San Diego, USA). Data acquisition and analysis were performed on a FACSCalibur flow cytometer (BD Bioscience) using FlowJo software (Tree Star Inc. Ashland, USA).
TGF-β1 and RA are well known to promote IgA switching of B cells (19,20,21,23,24). Recently, we observed that LF also caused spleen B cells to commit IgA class switch recombination (CSR) (31). Since TGF-β1 and RA increases IgA CSR in peritoneal B cells (25,26), we investigated whether LF possesses such an effect. We first examined the effect of LF, along with TGF-β1 and RA, on Ig production by mouse whole peritoneal B cells. LF, like TGF-β1, increased production of IgA, IgG2b, and IgG3 isotypes, whereas RA had little effect (Fig. 1). IgG1 production was marginal under any conditions (data not shown). These results indicate that LF can modulate peritoneal B cells to secrete IgA isotype.
Since LF strongly stimulated whole peritoneal B cells to secrete Igs, we compared Ig production between peritoneal B1 and B2 cell population. In peritoneal B1 cells, both LF and TGF-β1 substantially enhanced IgA and IgG3 production, but not IgG2b (Fig. 2). Interestingly, the results were opposite in peritoneal B2 cells: LF and TGF-β1 enhanced secretion of IgG2b, but not of IgA and IgG3. These results indicate that peritoneal B1 cells are rather specialized to produce IgA production under the influence of either LF or TGF-β1.
Thus far, LF acted like TGF-β1 in the regulation of Ig synthesis by peritoneal B cells. We and others have recently shown that TGF-β1, in combination with RA, enhances IgA production by splenic B cells and peritoneal B1 cells (19,20,21,23,24,26). Therein, we examined the effect of combination of LF and RA on Ig secretion by peritoneal B1 and B2 cells. As predicted, RA further augmented LF-induced IgA production by peritoneal B1 cells (Fig. 3). Again, under the same conditions, level of IgA production were lower in peritoneal B2 cells than in peritoneal B1 cells. Interestingly, this combination effect was confined to only IgA production. Any combined effect of LF and RA was not observed in other isotypes though LF increased production of IgG2b and IgG3 iso types (Fig. 3). These results indicate that LF and RA in combination can specifically modulate peritoneal B1 cells to produce IgA Ab.
Since peritoneal B1 cells produced IgA isotype preferentially, we suspect if B1 cells are already switched to IgA B cells more frequently than B2 cells. To assess this possibility, we determined surface IgA expression on freshly isolated peritoneal B1 and B2 cells. However, the sorted peritoneal B1 and B2 cells showed similar level of surface IgA expression (Fig. 4). These results imply that peritoneal B1 cells are readily switched to IgA B cells upon LF stimulation.
It has been demonstrated that peritoneal B1 cells contribute, in a T cell-independent manner, to at least half of the IgA plasma cells in the gut LP (16,33). RA is known to induce the expression of these molecules on B and T cells (34,35). Therefore, it was necessary to ask if LF and RA can regulate the expression of gut homing molecules (α4β7 and CCR) on peritoneal B1 cells. Consistent with the earlier findings, RA increased the expression of α4β7 and CCR9 on the surface of peritoneal B1 and B2 cells (Fig. 5). Here, double positive cells (α4β7+, CCR9+) were more frequent in peritoneal B1 cells than B2 cells. LF alone did not significantly affect the expression of either molecule. However, LF plus RA markedly enhanced the expression of α4β7 and CCR9 on peritoneal B1 cells, while only α4β7 on peritoneal B2 cells. Taken together, these results indicate that LF combined with RA preferentially induces IgA isotype switching and the expression of gut-homing molecules in peritoneal B1 cells.
The present study demonstrates that LF stimulates peritoneal B1 cells to produce IgA Ab. More importantly, when combined with RA, LF further enhanced IgA response and this increase was restricted to IgA isotype. Prior to this study, TGF-β1, a well-known IgA switching factor, has been shown to stimulate peritoneal B1 cells and splenic MZ B cells to increase IgA responses (25). They observed that peritoneal B1 and splenic MZ B cells are more responsive to TGF-β1 than peritoneal B2 and splenic FO B cells toward IgA expression. We recently found that LF can induce IgA production in mouse spleen MZ B cells and that it causes IgA CSR through direct interaction with betaglycan (TGF-β receptor III, Tβ RIII) and formation of the TβRIII/TβRII/TβRI complex (31). Therefore, it is likely that LF, like TGF-β1, can stimulate peritoneal B1 cells to commit IgA class switching. Nonetheless, it remains to determine if LF actually causes IgA CSR in peritoneal B1 cells at the molecular level.
One of the important activities of RA is to induce the expression of gut-homing molecules α4β7 and CCR9 on T and B cells (28,35). We have demonstrated that RA, in combination with TGF-β1, further enhances expression of α4β7 and CCR9 on B cells (24). Moreover, this effect is confined to peritoneal B1 and splenic MZ B cells (26). In the present study, we found that RA strongly induces the expression of α4β7 and CCR9 on peritoneal B1 cells while only α4β7 expression on peritoneal B2 cells. Herein, the RA-induced CCR9/α4β7 expression on peritoneal B1 cells was further augmented by LF treatment. Under the same conditions, only the expression of α4β7 increased on the surface of peritoneal B2 cells. These results suggest that, in the peritoneal cavity, only peritoneal B1 cells are selectively recruited to the gut, which is strongly stimulated by LF and RA. This is supported by several reports indicating that significant amount of gut IgA is derived from peritoneal B1 cells (15,16,17,18).
In conclusion, the present study demonstrates that peritoneal B1 cells, under the influence of LF and RA, do facilitate a prompt IgA response and the expression of gut-homing molecules. In this regard, LF and RA would be strong candidates for use as peritoneal adjuvants toward mucosal IgA response. Their use would be safe because these molecules are endogenously produced in body.
Figures and Tables
 | Figure 1Effect of LF, RA, and TGF-β1 on Ig secretion by mouse peritoneal B cells. Mouse whole peritoneal B cells were stimulated with LPS (12.5 µg/ml), RA (25 nM), LF (60 µg/ml), and TGF-β1 (0.2 ng/ml) for 7 days. Supernatants were collected, and Ig production was determined by isotype-specific ELISA. Data are means of triplicate samples±SEM. *p<0.05. |
 | Figure 2Effect of LF, RA, and TGF-β1 on Ig secretion by mouse peritoneal B1 and B2 cells. Mouse peritoneal B1 and B2 cells were stimulated with LPS (12.5 µg/ml), RA (25 nM), LF (60 µg/ml), and TGF-β1 (0.2 ng/ml) for 7 days. Supernatants were collected, and Ig production was determined by isotype-specific ELISA. Data represent the results of one of the two independent experiments and are means of triplicated samples±SEM. *p<0.05. |
 | Figure 3Combined effect of LF and RA on Ig secretion by mouse peritoneal B1 and B2 cells. Mouse peritoneal B1 and B2 cells were stimulated with LPS (12.5 µg/ml), RA (25 nM), and LF (30, 60, 120, 240 µg/ml in the serial dilution exp.; 60 µg/ml in the combination exp.) for 7 days. Supernatants were collected, and Ig production was determined by isotype-specific ELISA. Data represent the results of one of the two independent experiments and are means of triplicated samples±SEM. *p<0.05. |
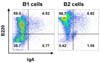 | Figure 4Measurement of membrane IgA on freshly isolated peritoneal B1 and B2 cells. Expression of mIgA and B220 was determined by FACS. |
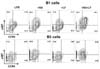 | Figure 5Effect of LF and RA on gut homing molecule expressed by peritoneal B1 and B2 cells. Mouse peritoneal B1 and B2 cells were cultured with LPS (12.5 µg/ml), RA (25 nM), and LF (60 µg/ml). After 4 days of culture, expression of surface CCR9 and α4β7 within B220 gated cell population was determined by FACS. Data represent the results of one of the two independent experiments. |
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2008536) and 2013 Research Grant from Kangwon National University (C1009704-01-01). Studies were carried out in the Institute of Bioscience and Biotechnology at Kangwon National University.
References
1. Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983; 157:202–218.

2. Gray D, MacLennan IC, Bazin H, Khan M. Migrant mu+ delta+ and static mu+ delta- B lymphocyte subsets. Eur J Immunol. 1982; 12:564–569.
3. Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset: strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989; 19:2163–2166.

4. Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013; 13:118–132.

5. Marcos MA, Huetz F, Pereira P, Andreu JL, Martinez A, Coutinho A. Further evidence for coelomic-associated B lymphocytes. Eur J Immunol. 1989; 19:2031–2035.

6. Kroese FG, Ammerlaan WA, Deenen GJ. Location and function of B-cell lineages. Ann N Y Acad Sci. 1992; 651:44–58.
7. Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991; 21:2951–2962.

8. Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992; 13:17–21.

9. Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001; 14:617–629.

10. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011; 11:34–46.

12. Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008; 1:11–22.

13. Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010; 28:243–273.

14. Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008; 28:740–750.

15. Suzuki K, Maruya M, Kawamoto S, Fagarasan S. Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunol Rev. 2010; 237:180–190.

16. Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989; 1:75–84.

17. Kroese FG, Butcher EC, Stall AM, Herzenberg LA. A major peritoneal reservoir of precursors for intestinal IgA plasma cells. Immunol Invest. 1989; 18:47–58.

18. Bos NA, Bun JC, Popma SH, Cebra ER, Deenen GJ, van der Cammen MJ, Kroese FG, Cebra JJ. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996; 64:616–623.

19. Coffman RL, Lebman DA, Shrader B. Transforming growth fact or beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989; 170:1039–1044.

20. Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989; 170:1415–1420.

21. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990; 145:3773–3778.
22. Tokuyama H, Tokuyama Y. Retinoids enhance IgA production by lipopolysaccharide-stimulated murine spleen cells. Cell Immunol. 1993; 150:353–363.

23. Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 1999; 192:41–47.

24. Seo GY, Jang YS, Kim HA, Lee MR, Park MH, Park SR, Lee JM, Choe J, Kim PH. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-beta1 to enhance the overall IgA response. J Leukoc Biol. 2013; 94:325–335.

25. Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J Immunol. 2006; 177:6025–6029.

26. Roy B, Brennecke AM, Agarwal S, Krey M, Duber S, Weiss S. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-beta and retinoic acid. PLoS One. 2013; 8:e82121.
27. Hart AL, Ng SC, Mann E, Al-Hassi HO, Bernardo D, Knight SC. Homing of immune cells: role in homeostasis and intestinal inflammation. Inflamm Bowel Dis. 2010; 16:1969–1977.

28. Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006; 314:1157–1160.

29. Lonnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009; 12:293–297.

30. Legrand D, Elass E, Carpentier M, Mazurier J. Interactions of lactoferrin with cells involved in immune function. Biochem Cell Biol. 2006; 84:282–290.
31. Jang YS, Seo GY, Lee JM, Seo HY, Han HJ, Kim SJ, Jin BR, Kim HJ, Park SR, Rhee KJ, Kim WS, Kim PH. Lactoferrin causes IgA and IgG2b isotype switching through betaglycan binding and activation of canonical TGF-beta signaling. Mucosal Immunol. 2014; doi: 10.1038/mi.2014.121.
32. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001; 31:1706–1715.

33. Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000; 288:2222–2226.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download