Abstract
Ginsenosides are the major components of ginseng, which is known to modulate blood pressure, metabolism, and immune function, and has been used to treat various diseases. It has been reported that ginseng and several ginsenosides have immunoregulatory effects on the innate and T cell-mediated immune response. However, their effects on the humoral immune response have not been fully explored. The present study examined the direct effects of red ginseng extract (RGE) and ginsenosides on mouse B cell proliferation and on antibody production and the expression of germline transcripts (GLT) by mouse B cells in vitro. RGE slightly reduced B cell proliferation, but increased IgA production by LPS-stimulated B cells. Furthermore, ginsenoside Rg1 and 20(S)-Rg3 selectively induced IgA production and expression of GLTα transcripts by LPS-stimulated B cells. Collectively, these results suggest that ginsenoside Rg1 and 20(S)-Rg3 can drive the differentiation of B cells into IgA-producing cells through the selective induction of GLTα expression.
Ginseng (Panax ginseng) has been widely used in traditional oriental tonic medicine for thousands of years to treat various diseases and to maintain body homeostasis. Many reports show that ginseng has multifunctional biological effects in immune function, anti-inflammatory, anti-cancer, anti-oxidant, metabolic processes (anti-diabetic) and the neuro-endocrine and cardiovascular system (blood pressure regulation) (123456). Ginseng contains many active ingredients including ginsenosides, polysaccharides, peptides, phytosterols, polyacetylenic alcohols and fatty acids (247). Among them, ginsenosides are known to have the most pharmacological and immunological activity (48). In the case of Korean ginseng, 38 ginsenosides have been identified and classified into three groups: protopanaxadiol (PPD), protopanaxatriol (PPT) and oleanane (4).
Recent investigations have demonstrated that ginsenosides are responsible for regulation of the immune response. It has been reported that ginsenoside Rg1 regulates the innate immune response in dendritic cells and macrophages by differentially modulating the production of inflammatory cytokines (910). Rg1 also increases CD4+ T cell activity and modulates Th1/Th2 differentiation in vitro and in vivo (1112). In addition, ginsenoside Rb1, Rd and Re elicit a Th1 and Th2 immune response (13141516), and recent studies have demonstrated that these ginsenosides (Rg1, Rb1, Rd, Re and Rg3) have immunological adjuvant activity to enhance the immune response (17181920212223).
Mature IgM+ B cells undergo Ig class switch recombination (CSR) at the switch region on the heavy chain locus to produce other Ig isotypes (IgG, IgA and IgE) and this class switching is selectively induced by cytokines such as IL-4, IFN-γ and TGF-β1 (24). In addition, expression of germline transcripts (GLTs) for each switch region is a prerequisite for each Ig CSR process (25). That is, the expression of GLα transcripts induces IgA CSR.
As mentioned above, ginsenosides act as adjuvants and then elicit both a humoral antibody response and a T cell mediated immune response. However, the direct effects of ginsenosides on the B cell response have not yet been investigated. To address this, we purified B cells from mouse splenocytes and examined the effects of red ginseng extract (RGE) and ginsenosides on B cell proliferation, antibody production, and expression of GLTs in vitro. Our study reveals that ginsenoside Rg1 and 20(S)-Rg3 selectively induce IgA production and GLTα expression by LPS-activated mouse B cells.
BALB/c mice were purchased from Damool Science (Daejeon, Korea) and maintained on an 8:16 h light:dark cycle in an animal environmental control chamber. Eight- to twelve-week-old mice were used, and animal care was in accordance with the institutional guidelines of the Institutional Animal Care and Use Committee of Konyang University.
Mouse splenic B cells were purified by positive selection of B220+ cells using anti-B220 microbeads or by depletion of CD43+ cells using anti-CD43 microbeads and high-gradient magnetic cell separation (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's instructions. Briefly, BALB/c mouse spleen cell suspensions were washed with HBSS (WelGENE, Daegu, Korea) and treated with 0.83% ammonium chloride to lyse the red blood cells. Spleen cells were treated with either anti-mouse B220 microbeads or anti-mouse CD43 microbeads and separated using a LS column and MACS Separator (Miltenyi Biotec, Auburn, CA, USA). The purity of B cells (≥98%) was assessed by FACSCalibur (BD Biosciences, San Jose, CA, USA) after staining the cells with anti-CD43 FITC (eBioscience, San Diego, CA, USA) and/or anti-B220 PE (BD Biosciences). Cells were cultured at 37℃ in a humidified CO2 incubator (Forma Scientific, Marietta, OH, USA) in RPMI-1640 medium (WelGENE) supplemented with 10% fetal bovine serum (PAA Laboratories, Etobicoke, ON, Canada). Purified B cells were stimulated with LPS (1 µg/ml, InvivoGen, San Diego, CA, USA; 12.5 µg/ml, Sigma-Aldrich, St Louis, MO, USA), red ginseng extract (200 µg/ml, Prepared by Dr. JE Choi, Chungnam National University, Daejeon, Korea) (26), ginsenoside Rb1, Rg1, 20(S)-Rg3 (each 2 µg/ml, Ambo Institute, Daejeon, Korea) and TGF-β1 (0.2 ng/ml, R&D Systems, Minneapolis, MN, USA).
Cell proliferation was determined using an EZ-Cytox cell viability assay kit (Daeil Lab Service Co, Seoul, Korea) as previously described (27). Briefly, 20 µl of EZ-Cytox kit reagent was added to each well of a 96-well microplate and then incubated at 37℃ in a humidified CO2 incubator for 3 h. After incubation, the optical density (OD) was measured at a wavelength of 450 nm using an Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Antibodies produced in B cell cultures were detected using isotype-specific ELISA. Affinity-purified anti-isotype specific antibodies were added at 1.2 µg/ml in 0.05 M sodium bicarbonate buffer (pH 9.5) to 96-well U bottomed polyvinyl microplates (Falcon, Becton Dickinson & Co., Oxnard, CA, USA). Plates were washed with PBS containing 0.05% Tween-20 (PBST) followed by overnight incubation at 4℃, and blocked for 1 h with 0.25% BSA solution. After washing, 50 µl of standard myeloma proteins and culture supernatants were added to each well and incubated for 1 h at 37℃. After washing, horseradish-peroxidase (HRPO) conjugated anti-isotype specific antibodies (Southern Biotechnology, Birmingham, AL, USA) were added to each well and incubated for 1 h. Plates were then washed, and TMB substrate (BD Biosciences) was added. After incubation, 0.05 M sulfuric acid was added to each well, and colorimetric reaction was measured at 450 nm with an Absorbance Microplate Reader.
RNA preparation, reverse transcription, and PCR were performed as previously described (28). The following PCR primers were synthesized by Bioneer (Daejeon, Korea): GLTα, forward 5'-CTACCATAGGGAAGATAGCCT-3', reverse 5'-TAATCGTGAATCAGGCAG-3' (product size, 206 bp); GLTγ1, forward 5'-CAGCCTGGTGTCAACTAG-3', reverse 5'-CTGTACATATGCAAGGCT-3' (product size, 532 bp); GLTγ2b, forward 5'-GGGAGAGCACTGGGCCTT-3', reverse 5'-AGTCACTGACTCAGGGAA-3' (product size, 318 bp); β-actin, forward 5'-CATGTTTGAGACCTTCAACACCCC-3', reverse 5'-GCCATCTCCTGCTCGAAGTCTAG-3' (product size, 320 bp). All reagents for RT-PCR were purchased from iNtRON Biotechnology (Seongnam, Korea). PCR for β-actin was performed in parallel to normalize the cDNA concentrations within each set of samples. Aliquots of the PCR products were resolved by electrophoresis on 2% agarose gels.
Red ginseng is usually produced by steaming ginseng root at 95~100℃ for 2~4 h, and then drying at 50~60℃ until the moisture content is <15% (29). In this study, we used two kinds of Korean red ginseng extracts (RGEs), which were prepared by water extraction (26): RGE1 was extracted at 75℃ for 24 h; RGE2 was extracted at 85℃ for 12 h. We first examined the effect of RGEs on B cell proliferation in vitro. As shown in Fig. 1A, both RGE1 and RGE2 substantially inhibited LPS-induced mouse splenic B cell proliferation. Next, we determined the effect of the RGEs on antibody production by B cells. The RGEs induced IgA production while decreasing other isotypes including IgM, IgG1 and IgG2b (Fig. 1B). RGE alone did not stimulate B cell proliferation or antibody production (data not shown). Thus, the RGEs selectively induced IgA production by LPS-stimulated mouse B cells. Further studies will be required to analyze the ginsenoside composition of the RGEs and to verify which ginsenoside(s) contributes to the selective induction of IgA production.
The most abundant ginsenosides in Korean red ginseng are Rb1 and Rg1 (4). In addition, Rg3 [20(R)-Rg3 and 20(S)-Rg3] is one of the most effective ginsenosides for cancer, inflammation, and obesity, and 20(S)-Rg3 is found only in red ginseng. It has been reported that ginsenoside Rb1, Rg1, and Rg3 regulate the T cell-mediated immune response (11121323). Therefore, we first examined whether Rb1, Rg1 and 20(S)-Rg3 have a direct effect on antibody production by mouse B cells. None of the ginsenosides alone were able to stimulate B cell proliferation or antibody production (data not shown). As shown in Fig. 2A, ginsenoside Rg1 and 20(S)-Rg3 significantly increased IgA production (1.7- and 1.9-fold increase, respectively, p <0.05) by LPS-stimulated B cells, while Rb1 had no significant effect on IgA production. Rg1, 20(S)-Rg3 and Rb1 did not increase IgM, IgG1 and IgG2b production. Rather, IgG1 production was decreased by Rb1 and Rg1. TGF-β1 is a well-known IgA and IgG2b class-switching factor (3031). We observed that TGF-β1 selectively enhances IgA and IgG2b production by LPS-activated B cells (Fig. 2A). All three ginsenosides had no effect on B cell proliferation (Fig. 2B). Furthermore, we observed that Rg1 and 20(S)-Rg3 selectively induce expression of GLTα in LPS-activated B cells (Fig. 2C). Our results show that, at least in part, ginsenoside Rg1 and 20(S)-Rg3 induce GLα transcription, resulting in IgA production by B cells.
In summary, in this report, we show for the first time that RGE and ginsenoside Rg1/(20)-S Rg3 can directly drive B cells to produce IgA. Further investigation is need to explore the mechanism(s) by which the ginsenosides induce GLα transcription. It will also be interesting to examine the direct effects of other ginsenosides, such as Rc, Rd and Rh1, on antibody production because it has been reported that intravenous injection of ginsenoside Rc and Rd differentially regulate antibody production (32) and ginsenoside Rh1 can alleviate inflammatory symptoms in atopic dermatitis via reduction of IgE (33).
Rats fed a ginsenoside diet had a significantly higher number of IgA+ cells in the jejunal lamina propria, suggesting that ginsenosides enhance IgA production and support a protective effect against mucosal infections (34). This, together with our results, suggests that ginsenosides [especially, Rg1 and 20(S)-Rg3] could be a selective adjuvant to enhance IgA production by B cells and could be used as potential agents for a mucosal vaccine.
Figures and Tables
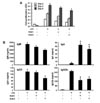 | Figure 1Effects of red ginseng extract on B cell proliferation and antibody production. Purified mouse splenic B cells were stimulated with LPS (1 µg/ml) and red ginseng extracts (RGE1 and RGE2; each 200 µg/ml). (A) After 2, 3 and 4 days of culture, cell proliferation (OD) was measured using an EZ-Cytox cell viability assay. Data are averages of two independent experiments with ranges (bars). (B) After 7 days of culture, supernatants were harvested, and levels of Igs production were determined using an isotype-specific ELISA. Data represent means±SEM of triplicate samples. *p<0.05. |
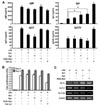 | Figure 2Effects of ginsenosides on antibody production and expression of germline transcripts. Purified mouse splenic B cells were stimulated with LPS (12.5 µg/ml), ginsenoside [Rb1, Rg1, and 20(S)-Rg3; each 2 µg/ml], and TGF-β1 (0.2 ng/ml). (A) After 7 days of culture, supernatants were harvested and levels of Igs production were determined using an isotype-specific ELISA. Data represent the average±SEM of three independent experiments. *p<0.05. (B) After 2 and 3 days of culture, cell proliferation (OD) was measured using an EZ-Cytox cell viability assay. Data are averages of two independent experiments with ranges (bars). (C) After 2.5 days of culture, RNAs were isolated, and GLTs and β-actin mRNA levels were measured by RT-PCR. Data are representative of two independent experiments. |
ACKNOWLEDGEMENTS
This work was supported by Konyang University Myunggok Research Fund of 2014. H.-Y.P. is an awardee of full research scholarships provided by Graduate School of Konyang University.
References
2. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693.
3. Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006; 11:128–150.
4. Choi KT. Botanical characteristics, pharmacological ef fects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008; 29:1109–1118.

5. Jia L, Zhao Y. Current evaluation of the millennium phytomedicine--ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009; 16:2475–2484.

8. Yue PY, Mak NK, Cheng YK, Leung KW, Ng TB, Fan DT, Yeung HW, Wong RN. Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin Med. 2007; 2:6.

9. Rhule A, Rase B, Smith JR, Shepherd DM. Toll-like receptor ligand-induced activation of murine DC2.4 cells is attenuated by Panax notoginseng. J Ethnopharmacol. 2008; 116:179–186.

10. Wang Y, Liu Y, Zhang XY, Xu LH, Ouyang DY, Liu KP, Pan H, He J, He XH. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-kappaB and PI3K/Akt/mTOR pathways. Int Immunopharmacol. 2014; 23:77–84.

11. Lee EJ, Ko E, Lee J, Rho S, Ko S, Shin MK, Min BI, Hong MC, Kim SY, Bae H. Ginsenoside Rg1 enhances CD4(+) T-cell activities and modulates Th1/Th2 differentiation. Int Immunopharmacol. 2004; 4:235–244.

12. Lee JH, Han Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int Immunopharmacol. 2006; 6:1424–1430.

13. Rivera E, Ekholm Pettersson F, Inganas M, Paulie S, Gronvik KO. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine. 2005; 23:5411–5419.

14. Yang Z, Chen A, Sun H, Ye Y, Fang W. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine. 2007; 25:161–169.

15. Son YM, Kwak CW, Lee YJ, Yang DC, Park BC, Lee WK, Han SH, Yun CH. Ginsenoside Re enhances survival of human CD4+ T cells through regulation of autophagy. Int Immunopharmacol. 2010; 10:626–631.

16. Kim J, Han BJ, Kim H, Lee JY, Joo I, Omer S, Kim YS, Han Y. Th1 immunity induction by ginsenoside Re involves in protection of mice against disseminated candidiasis due to Candida albicans. Int Immunopharmacol. 2012; 14:481–486.

17. Sun J, Song X, Hu S. Ginsenoside Rg1 and aluminum hydroxide synergistically promote immune responses to ovalbumin in BALB/c mice. Clin Vaccine Immunol. 2008; 15:303–307.

18. Qu DF, Yu HJ, Liu Z, Zhang DF, Zhou QJ, Zhang HL, Du AF. Ginsenoside Rg1 enhances immune response induced by recombinant Toxoplasma gondii SAG1 antigen. Vet Parasitol. 2011; 179:28–34.

19. Su F, Yuan L, Zhang L, Hu S. Ginsenosides Rg1 and Re act as adjuvant via TLR4 signaling pathway. Vaccine. 2012; 30:4106–4112.

20. Han Y, Rhew KY. Ginsenoside Rd induces protective anti-Candida albicans antibody through immunological adjuvant activity. Int Immunopharmacol. 2013; 17:651–657.

21. Song X, Chen J, Sakwiwatkul K, Li R, Hu S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int Immunopharmacol. 2010; 10:351–356.

22. Su X, Pei Z, Hu S. Ginsenoside Re as an adjuvant to enhance the immune response to the inactivated rabies virus vaccine in mice. Int Immunopharmacol. 2014; 20:283–289.

23. Wei X, Chen J, Su F, Su X, Hu T, Hu S. Stereospecificity of ginsenoside Rg3 in promotion of the immune response to ovalbumin in mice. Int Immunol. 2012; 24:465–471.

25. Stavnezer J. Molecular processes that regulate class switching. Curr Top Microbiol Immunol. 2000; 245:127–168.

26. Lee GS, Nam KY, Choi JE. Ginsenoside composition and quality characteristics of red ginseng extracts prepared with different extracting methods. Korean J Med Crop Sci. 2013; 21:276–281.

27. Kim YH, Lee SH, Yoo YC, Lee J, Park JH, Park SR. Kinetic analysis of CpG-induced mouse B cell growth and Ig production. Immune Netw. 2012; 12:89–95.

28. Park SR, Kim PH, Lee KS, Lee SH, Seo GY, Yoo YC, Lee J, Casali P. APRIL stimulates NF-kappaB-mediated HoxC4 induction for AID expression in mouse B cells. Cytokine. 2013; 61:608–613.

29. Gui Y, Ryu GH. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder). J Ginseng Res. 2014; 38:146–153.

30. Lebman DA, Nomura DY, Coffman RL, Lee FD. Molecular characterization of germ-line immunoglobulin A transcripts produced during transforming growth factor type beta-induced isotype switching. Proc Natl Acad Sci USA. 1990; 87:3962–3966.

31. McIntyre TM, Klinman DR, Rothman P, Lugo M, Dasch JR, Mond JJ, Snapper CM. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med. 1993; 177:1031–1037.

32. Mita A, Shida R, Kasai N, Shoji J. Enhancement and suppression in production of IgM-antibody in mice treated with purified saponins. Biomedicine. 1979; 31:223–227.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download