Abstract
Inflammation is the basis of severe acute and chronic diseases. This study investigated the anti-inflammatory property of a crude methanol extract (MeOH-ex) and the solvent fractions of Ixeris dentata Nakai (IDN) in LPS-stimulated murine macrophage-like cell line RAW264.7. Here, we showed that the ethyl acetate fraction (EtOAc-fr) had the most potent inhibitory activity on LPS-induced nitric oxide (NO) production among the tested samples, i.e., IDN MeOH-ex and the three different solvent fractions (chloroform, n-hexane, and EtOAc). We further found that the EtOAc-fr significantly inhibited LPS-induced prostaglandin PGE2 (PGE2) generation in RAW264.7 cells. Furthermore, the treatment with EtOAc-fr effectively suppressed the expression of inducible NO synthase (iNOS) and cyclooxygenase 2 (COX-2). These results suggest that the EtOAc-fr of IDN MeOH-ex exhibits an anti-inflammatory activity in vitro by inhibiting LPS-induced NO production and PGE2 generation via suppression of iNOS and COX-2 expression.
Inflammation, defined as the immune system response to injury or infection, is a protective response that leads to the removal of initiating noxious stimuli or offending factors and the restoration of tissue structures and physiological functions. However, excessive chronic inflammation represents the basis of severe diseases including inflammatory bowel disease, arthritis, asthma, cancer, atherosclerosis, Alzheimer's disease, and Parkinson's disease (12345). Therefore, controlling the inflammatory responses is important to prevent and treat many diseases. Nitric oxide (NO), a gaseous free radical, and prostaglandin E2 (PGE2), an eicosanoid derived from arachidonic acid, have been identified as important biological molecules involved in the immune responses and inflammation (67). Upon inflammatory stimulation, large amounts of NO and PGE2 produced by inducible NO synthases (iNOS) and cyclooxygenase 2 (COX-2), respectively, induce substantial inflammatory response and related processes (68). Therefore, the regulation of NO production and PGE2 generation is the "gold standard" for the prevention and treatment of many inflammatory diseases.
Ixeris dentata Nakai (IDN) has been used as a traditional herbal medicine in East Asian countries to treat indigestion, pneumonia, hepatitis, contusions, and tumors (9). Previous studies reported that IDN exhibits multiple pharmacological activities including antidiabetic, anticolitic, antiallergic, and neuroprotective properties (101112131415). Furthermore, it has been reported that the IDN water extract possesses an anti-inflammatory activity against 2,4-dinitrofluorobenzene- induced atopic dermatitis-like skin inflammation (16). Previous studies demonstrated that several biologically active compounds including sesquiterpene lactones and flavonoids isolated from Ixeris dentate possesses multiple pharmacological activities on cancer (917), acylcoenzyme A: cholesterol acyltransferase (ACAT) activity (9), and inflammation (131819). While the anti-inflammatory activities of IDN and its bio-active compounds have been reported previously, the effect of IDN on the production of pro-inflammatory mediators including PGE2 and NO has not yet been fully studied. Thus, in the present study, we evaluated the inhibitory properties of IDN methanol extract (MeOH-ex) and its solvent fractions on NO production and PGE2 generation in a murine macrophagelike cell line RAW264.7, which can be activated with LPS to induce pro-inflammatory mediators.
The IDN was purchased from the local herbal market (Asan, Chungnam, Korea) and verified at the Department of Plant Resources, Soonchunhyang University (Asan, Chungnam, Korea). The air-dried IDN (2 kg) was powdered and exhaustively extracted with 99.8% MeOH (3×1.5 L) at room temperature for 48 h. The solution was filtered and concentrated under reduced pressure on a rotatory evaporator at 45℃, resulting in 205.8 g of crude MeOH-ex. The entire MeOH-ex (200 g) was suspended in 0.5 L of water and then partitioned sequentially with equal volumes of hexane (n-hexane), chloroform (CHCl3), and ethyl acetate (EtOAc). Each fraction was evaporated in vacuo to yield the residues of n-hexane, CHCl3, and EtOAc fractions. The MeOH-ex and the three fractions were tested for inhibitory effects against NO and PGE2 generation by using a murine macrophage cell line, RAW264.7.
RAW264.7 cells, obtained from American Type Culture Collection (Manassas, VA, USA), were cultured in DMEM (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated FBS, 100 U/mL of penicillin, and 100 µg/mL streptomycin at 37℃ under humidified air containing 5% CO2 inside an incubator. Cells were plated in 35-mm culture dishes at a density of 6×105 cells/dish for the following experiments.
RAW264.7 cells were plated at a density of 2×104 cells/well in 96-well plates, and then stimulated with or without LPS (1 µg/mL) in the absence or presence of various concentrations of testing samples for 24 h. The nitrite accumulation in the supernatant was assessed by the Griess reaction (20). Each 50 µL of culture supernatant was mixed with an equal volume of Griess reagent (0.1% N-(1-naphthyl)- ethylenediamine and 1% sulfanilamide in 5% phosphoric acid) and incubated at room temperature for 10 min. The absorbance at 550 nm was measured using an automated microplate reader and a series of known concentrations of sodium nitrite was used as the standard.
RAW264.7 cells were plated at a density of 2×104 cells/well in 96-well plates, and then stimulated with or without LPS (1 µg/mL) in the absence or presence of various concentrations of testing samples for 24 h. Vehicle (DMSO) only cultures served as controls. Cell culture supernatants were harvested and the generation of PGE2 in cell culture was measured using commercial ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
The cytotoxic effect of the various samples after 24 h of incubation was evaluated in the cells using the MTT assay to determine the appropriate concentration that is not cytotoxic to the cells. Briefly, RAW264.7 cells were plated at a density of 2×104 cells/well in 96-well plates and then treated with various concentrations of testing sample. Following incubation for 24 h, the culture medium was removed and replaced with 100 µL of fresh medium, and then 20 µL of 0.5 mg/mL MTT solution was added to each well. After incubation for 1.5 h, the medium with MTT was removed and 200 µL of DMSO was added to each well. The plates were then gently agitated until the color reaction was uniform and the colorimetric evaluation was performed with a microplate reader at 540 nm.
Whole cell lysates were obtained as previously described (21). SDS-PAGE and western blot analysis were performed as described previously (2122). Briefly, the cells were cultured in 6-well culture plates at a density of 5×105 cells/well for 24 h. The cells were then stimulated with or without LPS (1 µg/mL) in the absence or presence of various concentrations of testing samples for 24 h. The cells were washed twice with PBS, lysed, and the proteins were separated on 10% to 15% SDS-PAGE. The proteins were transferred to polyvinylidene fluoride (PVDF) membrane, and membranes were blocked with 5% skim milk in TBST-buffer for 2.5 h at room temperature. The protein-transfer membranes were probed with the following primary antibodies: mouse monoclonal antibodies directed against rabbit polyclonal antibodies directed against iNOS (1:500, Santa Cruz Biotechnology, Dallas, TX, USA) and COX-2 (1:500, Santa Cruz Biotechnology). Protein expression was visualized using a chemiluminescence reagent (Amersham Pharmacia Biotech, Inc., Buckinghamshire, UK), and detected using a digital chemiluminescence imaging system equipped with a charge coupled device (CCD) camera (Fusion-FX, Fisher BioTec Ltd., Wembley, Australia).
Statistical analyses were carried out using the GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). Pairwise comparisons were performed using oneway ANOVA Dunnett's tests. Data are presented as mean±SEM in the indicated number of experiments. The differences between groups were considered significant at p-value below 0.05.
One hallmark of chronic inflammation is the continuous recruitment and activation of macrophages to the sites of inflammation. Macrophages play a central role in inflammatory responses through phagocytosis, antigen presentation, and immunomodulation (23). Activated macrophages produce a wide variety of pro-inflammatory mediators, such as NO and PGE2 which are generated by iNOS and COX-2, respectively (24). Excessive release of these pro-inflammatory mediators from activated macrophages has long been recognized as a risk factor for inflammatory diseases (25). To evaluate the anti-inflammatory property of IDN, we initially evaluated the effect of IDN MeOH-ex and its solvent fractions on LPS-induced NO production in murine macrophage-like cell line, RAW264.7. In contrast to the LPS control group, IDN MeOH-ex treatment significantly inhibited the LPS-induced NO production in a concentration-dependent manner (Fig. 1A). We further found that treatment with n-hexane fraction (n-hexane-fr, Fig. 1B), CHCl3 fraction (CHCl3-fr, Fig. 1C), and EtOAc fraction (EtOAc-fr, Fig. 1D) effectively inhibited NO production in a concentration-dependent manner with IC50 values of 102.6, 87.8, and 16.4 µg/mL, respectively. In addition, we observed that the LPS-induced PGE2 generation in RAW264.7 cells was also significantly decreased by n-hexane-fr (Fig. 2B), CHCl3-fr (Fig. 2C), and EtOAcfr (Fig. 2D) with IC50 values of 94.4, 123.7, and 85.8 µg/mL, respectively, while MeOH-ex showed a non-dose-dependent inhibitory activity (Fig. 2A). Our results indicated that the IDN EtOAc-fr has a potent inhibitory activity on LPS-stimulated inflammatory responses in macrophages. Previous studies have also reported that TNF-α, IL-6, and IL-1β levels were significantly lowered by IDN water extract treatment in acute colitis and dermatitis (1216). Taken together, these results suggested that the IDN extract possessed an effective anti-inflammatory property against multiple inflammatory responses.
Since EtOAc-fr was identified to have the most potent anti-inflammatory property against LPS-induced inflammation, we further evaluated its anti-inflammatory mechanism. Previously, it has been demonstrated that treatment with IDN water extract effectively reduced the expression of pro-inflammatory proteins, such as NF-κB and MAPKs (121618), but the effects of IDN MeOH-ex and its solvent fractions on the expression of pro-inflammatory proteins regulating NO production and PGE2 generation have not been extensively investigated. To investigate the possible mode of action, we tested the effect of IDN EtOAc-fr on iNOS and COX-2 expression in LPS-stimulated RAW264.7 cells. Stimulation with LPS upregulated the expression of iNOS and COX-2 in cells compared with the unstimulated controls. We found that treatment of cells with IDN EtOAc-fr significantly suppressed LPS-induced iNOS expression in a dose-dependent manner (Fig. 3A). This result suggests that the IDN EtOAc-fr inhibits LPSinduced NO production and PGE2 generation through suppression of iNOS and COX-2 expression. To examine whether the anti-inflammatory activity of IDN EtOAc-fr in RAW264.7 cells is attributable to its cytotoxicity, we examined the effect of IDN EtOAc-fr treatment on cell viability. Importantly, an MTT assay using RAW264.7 cells treated with IDN EtOAc-fr alone (up to 200 µg/mL) showed that the cell morphology (Fig. 3D) and cell viability (Fig. 3E) were not significantly altered. These results demonstrated that IDN EtOAc-fr exhibited anti-inflammatory activity without affecting cell viability.
In conclusion, we identified the EtOAc-fr of IDN MeOH-ex as an anti-inflammatory substance that effectively inhibited LPS-induced NO production and PGE2 generation by suppressing iNOS and COX-2 expressions in RAW264.7 cells. Therefore, the bioactive components of IDN EtOAc-fr may be promising natural anti-inflammatory compounds for the prevention and treatment of multiple inflammatory diseases. Further studies are required to identify the bioactive compounds of IDN.
Figures and Tables
 | Figure 1The effect of a crude methanol extract and solvent fractions of Ixeris dentata Nakai (IDN) on nitric oxide production in LPS-stimulated RAW264.7 cells. Cells were treated with a range of concentrations of (A) IDN crude methanol extract (MeOH-ex), (B) normal hexane fraction (n-hexane-fr), (C) chloroform fraction (CHCl3-fr), and (D) ethyl acetate fraction (EtOAcfr) or vehicle (DMSO, white and black bars) for 24 h, as presented in the graphs. The production of nitric oxide (NO) in cells stimulated with 1 µg/mL of LPS was determined. Data are expressed as mean±SEM of three individual experiments with triplicate of each experiment. #p<0.05 vs. normal control, and *p<0.05 vs. vehicle-treated control. |
 | Figure 2The effect of a crude methanol extract and solvent fractions of Ixeris dentata Nakai (IDN) on prostaglandin E2 generation in LPS-stimulated RAW264.7 cells. Cells were treated with a range of concentrations of (A) IDN crude methanol extract (MeOH-ex), (B) normal hexane fraction (n-hexane-fr), (C) chloroform fraction (CHCl3-fr), and (D) ethyl acetate fraction (EtOAcfr) or vehicle (DMSO, white and black bars) for 24 h, as presented in the graphs. The generation of prostaglandin E2 (PGE2) in cells stimulated by 1 µg/mL of LPS was determined. Data are expressed as mean±SEM of three separated experiments with triplicate of each experiment. #p<0.05 vs. normal control, and *p<0.05 vs. vehicle-treated control. |
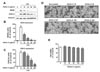 | Figure 3The effect of ethyl acetate fraction (EtOAc-fr) of Ixeris dentata Nakai (IDN) crude methanol extract on the expression of iNOS and COX-2, and viability of RAW264.7 cells. Cells were treated with a range of concentrations of EtOAc-fr of MeOH-ex for 24 h. (A) The protein expression of iNOS and COX-2 were determined using western blot analysis. Blots were quantitated by Image J software. The graphs show the relative expression level of (B) iNOS and (C) COX-2 normalized to β-actin levels. Cell morphological changes (D) and viability (E) after treatment with EtOAc-fr or vehicle (DMSO) for 24 h were examined, and the results are expressed as percentages relative to the vehicle (DMSO)-treated control (white bar). Data are expressed as mean±SEM of three separated experiments with triplicate of each experiment. #p<0.05 vs. normal control, and *p<0.05 vs. vehicle-treated control. |
References
1. Guzik TJ, Korbut R, mek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003; 54:469–487.
2. Lee TH, Song HJ, Park CS. Role of inflammasome activation in development and exacerbation of asthma. Asia Pac Allergy. 2014; 4:187–196.

3. Selmi C, Generali E, Massarotti M, Bianchi G, Scire CA. New treatments for inflammatory rheumatic disease. Immunol Res. 2014; 60:277–288.

4. Su X, Federoff HJ. Immune responses in Parkinsons disease: interplay between central and peripheral immune systems. Biomed Res Int. 2014; 2014:275178.

5. Zhiqin W, Palaniappan S, Raja Ali RA. Inflammatory Bowel Disease-related colorectal cancer in the Asia-Pacific Region: Past, Present, and Future. Intest Res. 2014; 12:194–204.

7. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011; 31:986–1000.

8. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002; 23:144–150.

9. Ahn EM, Bang MH, Song MC, Park MH, Kim HY, Kwon BM, Baek NI. Cytotoxic and ACAT-inhibitory sesquiterpene lactones from the root of Ixeris dentata forma albiflora. Arch Pharm Res. 2006; 29:937–941.

10. Oh SH, Sung TH, Kim MR. Ixeris dentata extract maintains glutathione concentrations in mouse brain tissue under oxidative stress induced by kainic acid. J Med Food. 2003; 6:353–358.

11. Lee HY, Lee GH, Kim HK, Kim SH, Park K, Chae HJ, Kim HR. Ixeris dentata-induced regulation of amylase synthesis and secretion in glucose-treated human salivary gland cells. Food Chem Toxicol. 2013; 62:739–749.

12. Kim DS, Ko JH, Jeon YD, Han YH, Kim HJ, Poudel A, Jung HJ, Ku SK, Kim SJ, Park SH, Park JH, Choi BM, Park SJ, Um JY, Hong SH. Ixeris dentata NAKAI reduces clinical score and HIF-1 expression in experimental colitis in mice. Evid Based Complement Alternat Med. 2013; 2013:671281.
13. Kim SB, Kang OH, Joung DK, Mun SH, Seo YS, Cha MR, Ryu SY, Shin DW, Kwon DY. Anti-inflammatory effects of tectroside on UVB-induced HaCaT cells. Int J Mol Med. 2013; 31:1471–1476.

14. Yi JM, Hong SH, Lee HJ, Won JH, Kim JM, Jeong DM, Baek SH, Lim JP, Kim HM. Ixeris dentata green sap inhibits both compound 48/80-induced aanaphylaxis-like response and IgE-mediated anaphylactic response in murine model. Biol Pharm Bull. 2002; 25:5–9.

15. Park EK, Sung JH, Trinh HT, Bae EA, Yun HK, Hong SS, Kim DH. Lactic acid bacterial fermentation increases the antiallergic effects of Ixeris dentata. J Microbiol Biotechnol. 2008; 18:308–313.
16. Jeon YD, Kee JY, Kim DS, Han YH, Kim SH, Kim SJ, Hong SH. Effects of Ixeris dentata water extract and caffeic acid on allergic inflammation in vivo and in vitro. BMC Complement Altern Med. 2015; 15:196.
17. Cha MR, Choi YH, Choi CW, Yoo DS, Kim YS, Choi SU, Kim YH, Ryu SY. New guaiane sesquiterpene lactones from Ixeris dentata. Planta Med. 2011; 77:380–382.

18. Karki S, Park HJ, Nugroho A, Kim EJ, Jung HA, Choi JS. Quantification of major compounds from Ixeris dentata, Ixeris dentata Var. albiflora, and Ixeris sonchifolia and their comparative anti-inflammatory activity in lipopolysaccharidestimulated RAW 2647 cells. J Med Food. 2015; 18:83–94.

19. Park S, Nhiem NX, Lee TH, Kim N, Kim SY, Chae HJ, Kim SH. Isolation of two new bioactive sesquiterpene lactone glycosides from the roots of Ixeris dentata. Bioorg Med Chem Lett. 2015; 25:4562–4566.

20. Nath J, Powledge A. Modulation of human neutrophil inflammatory responses by nitric oxide: studies in unprimed and LPS-primed cells. J Leukoc Biol. 1997; 62:805–816.

21. Son DJ, Ha SJ, Song HS, Lim Y, Yun YP, Lee JW, Moon DC, Park YH, Park BS, Song MJ, Hong JT. Melittin inhibits vascular smooth muscle cell proliferation through induction of apoptosis via suppression of nuclear factor-kappaB and Akt activation and enhancement of apoptotic protein expression. J Pharmacol Exp Ther. 2006; 317:627–634.

22. Ban JO, Yuk DY, Woo KS, Kim TM, Lee US, Jeong HS, Kim DJ, Chung YB, Hwang BY, Oh KW, Hong JT. Inhibition of cell growth and induction of apoptosis via inactivation of NF-kappaB by a sulfurcompound isolated from garlic in human colon cancer cells. J Pharmacol Sci. 2007; 104:374–383.

23. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286.

24. van Hoogmoed LM, Harmon FA, Stanley S, White J, Snyder J. In vitro investigation of the interaction between nitric oxide and cyclo-oxygenase activity in equine ventral colon smooth muscle. Equine Vet J. 2002; 34:510–515.

25. Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011; 13:11–22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download