Abstract
The nucleotide-oligomerization domain (NOD) is an important molecule involved in host defense against bacterial infection. To study the role of NODs in the host response to Mycobacterium leprae, we measured the mRNA levels of NODs and related genes in infected mouse tissues. The mRNA expression of NOD1, NOD2, caspase-1 and ASC was increased in mouse footpads. Whereas NOD2 expression in macrophages was increased at 2 and 24 h post-infection with M. leprae, there was no expression of NOD1 at these time points. An increase in caspase-1 expression was observed at 2 h and continued at 24 h. However, the expression of ASC was increased only at the early time point. The expression of caspase-1 is regulated by NOD2-dependent pathway in established HEK 293. Our results suggest NOD2, rather than NOD1, may be associated with the host response to M. leprae and that caspase-1 activation is essential for the host response.
Mycobacterium leprae is an intracellular pathogen that resides and replicates in phagocytes such as macrophages. M. leprae can induce phagocytes to produce inflammatory cytokines, such as TNF-α and IL-12, which are involved in the control of bacterial replication and the coordination of adaptive immune responses (12).
Pattern recognition receptors (PRRs) are essential components for probing pathogen infection and evoking production of pro-inflammatory cytokines in the innate immune system. PRRs include toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) (3). NLRs are cytosolic receptors that are involved in manifold biological processes, including host defense against pathogens (45).
NOD1 and NOD2 are a subfamily of NLRs and their stimulation leads to activation of the nuclear transcription factor (NF)-κB, extracellular signal-regulated kinase (ERK) and Jun N-terminal kinase (JNK), which are known to be triggered by mycobacteria (67). This activation results in the expression of pro-inflammatory molecules that induce both innate and adaptive immune responses. NOD2 also induces caspase-1 activation (8), which mediates the maturation of pro-IL-1β to its active form, IL-1β (9).
NODs recognize bacterial molecules produced during the synthesis and degradation of peptidoglycan. In particular, NOD2 is activated by muramyl dipeptide (MDP), a component of peptidoglycan (PGN) (101112). NOD2 has also been implicated in sensing intracellular pathogens such as Listeria monocytogenes (6) and M. tuberculosis (13). However, despite their importance, the role of NODs in M. leprae infection has not been elucidated.
The inflammasome is a protein complex consisting of a nucleotide-binding domain, a leucine-rich repeats-containing family, a pyrin domain-containing (NLRP), apoptotic speck protein containing a caspase recruitment domain (ASC) PYCARD and caspase-1, and is a component of the innate immune system. The inflammasome promotes maturation of the inflammatory cytokine IL-1β and is responsible for activation of an inflammatory reaction (1415).
To date, many studies of NODs have focused on the secretion of pro-inflammatory cytokine such as IL-1β through binding of their ligands, but there is no such evidence for their role in mycobacterial disease, particularly in leprosy. In this study, we assess the role of NODs in the host response to M. leprae infection by measuring the mRNA levels of NODs and related genes in infected mouse tissues.
The use of M. leprae-infected mice for the preparation of M. leprae was approved by the Department of Laboratory Animal IACUC in Songeui Campus. M. leprae was prepared from the foot-pads of M. leprae-infected BALB/c nude mice. Foot-pads were treated with Potadine solution and washed with ice-cold DPBS to remove exogenous contamination. To isolate M. leprae, the foot-pads were excised, cut into small pieces, and ground with a MACs isolator (Miltenyl biotec. Germany). The extract was filtered using a Cell strainer (BD Falcon, Durham, NC, USA) to remove tissue debris and centrifuged for 20 min at 4℃. The pellet was resuspended in 1 ml of ice-cold DPBS, and treated with 2N sodium hydroxide for 5 min to remove tissue-derived cells. The reaction was neutralized by adding 13 ml of ice-cold DPBS. After centrifugation and resuspension, acid-fast staining was performed, and bacteria were counted under light microscopy (1,000×oil-lens) using the procedure of Shepard and McRae (16).
The human embryonic kidney HEK 293 and murine RAW 264.7 cell lines were purchased from the American Type Culture Collection. The cell lines were cultured in DMEM (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone) and antibiotics (Gibco, Grand Island, NY, USA). The cells were infected with M. leprae at a multiplicity-of-infection (MOI) of 10. RAW cells were also stimulated with LPS (derived from E. coli O111:B4, Sigma-Aldrich, St. Louis, USA). In some experiments, the cells were incubated with caspase-1 inhibitor (Ac-YVAD-CMK, Calbiochem, Darmstadt, Germany) 1 h before infection.
Culture supernatants were assayed for mouse IL-1β by ELISA (DuoSet, R&D, Minneapolis, MN, USA) according to manufacturer protocols. Using this assay, the lower limit of detection of IL-1β was 3.9 pg/ml. Cell lysates were centrifuged at 10,000 g for 5 min at 4℃ and caspase-1 activity assay was performed using a caspase-1 assay kit (Calbiochem) as previously described (1718). The total increase in the optical density at 405 nm versus that of the sample alone was calculated. Caspase-1 activity was expressed as: (maximum OD405/microgram protein)×10,000.
Human embryonic kidney (HEK) 293T cells were plated into 12-well plates (BD Falcon) at 2×105 cells/well and incubated overnight in a CO2 incubator. The cells were co-transfected with varying amounts of pcDNA3-NOD1, pcDNA3-NOD2, or pcDNA3-NOD1-NOD2. The transfection was performed with transfection reagent (FuGENE HD, Roche, Switzerland) according to the manufacturer's instruction. The transfected cells were washed and placed in serum-free DMEM and stimulated with M. leprae (MOI of 10).
Total RNA from M. leprae-infected or non-infected tissues and cells was prepared using TRIzol reagent (Invitrogen, Waltham, MA, USA) and treated with DNase I (Qiagen, Valencia, CA, USA) to remove any contaminating genomic DNA. The amount of total RNA was quantified with spectrophotometer. cDNA was synthesized using a SuperScript cDNA synthesis III kit (Invitrogen) according to manufacturer instructions.
Quantitative RT-PCR was used to detect Caspase-1, Nod1, Nod2 and ASC transcripts in mouse footpads and RAW264.7 cells. β2M was used as an endogenous control. PCR amplification was performed with 2×QantiTect SYBR Green PCR Master mix (Qiagen) with validated primers (Qiagen) according to manufacturer protocols. The PCR conditions were as follows: 95℃ for 15 min, followed by 40 cycles of 95℃ for 15 s, 55℃ (Caspase1, Nod1, Nod2, ASC, and β2M) for 30 s and 72℃ for 30 s. Levels of mRNA were measured using Chromo 4 (MJ Research, Waltham, MA, USA). For relative quantification, the expression of each gene was normalized to the expression of β2M in the cells relative to a calibrator. The amount of target was represented as 2-ΔΔCt. The primers used in this study are shown in Table I.
Statistical analyses were carried out using SigmaStat, version 3.1, by one-way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA, depending on the data. The significance was further confirmed by the Tukey test. Differences were considered significant when p was less than 0.05.
Our previous report showed that M. leprae induced caspase-1 activation and IL-1β production in peritoneal cells from C57BL6 mice (19). In the present study, RAW 264.7 cells (a macrophage cell line) were exposed to M. leprae overnight, and the levels of IL-1β production and caspase-1 activity were measured. Consistent with our previous results, M. leprae bacilli induced caspase-1 activation and a low level of IL-1β production in RAW cells (Fig. 1). In contrast, LPS induced higher levels of IL-1β production under the same conditions, suggesting that the low level of IL-1β production was due to the doubling time of M. leprae. To determine whether caspase-1 is essential for IL-1β secretion in M. leprae infection, RAW cells were incubated with the caspase-1 inhibitor Ac-YVAD-CMK for 1 h before infection, and then caspase-1 activity and IL-1β secretion were measured. The M. leprae-induced IL-1β secretion was blocked in cells that lacked caspase-1 (Fig. 1A and B), suggesting that caspase-1 activity is necessary for IL-1β secretion in response to M. leprae infection.
Previous reports have shown that NOD2 induces caspase-1 activation, and that NOD signaling has a dual effect by activating proIL-1β mRNA transcription and inducing the release of bioactive IL-1β (8). To assess the role of NODs in the host response to M. leprae infection, we examined the expression of NODs and related genes in mouse tissues infected with M. leprae, and compared that to non-infected tissues. We first measured the mRNA expression of NOD1, NOD2, caspase-1 and ASC in footpads from nude mice infected with M. leprae for 18 months. M. leprae infection increased the expression of both NOD1 and NOD2 mRNA. The mRNA expression of caspase-1 and ASC, which are components of the inflammasome, was also induced by M. leprae infection (Fig. 2).
Next, we investigated NOD expression in RAW 264.7 cells after M. leprae infection. Compared to uninfected cells, mRNA levels for NOD1 and NOD2 were increased at 1 h after infection with M. leprae. Whereas NOD2 expression was increased at 2 and 24 h post-infection with M. leprae, while the expression of NOD1 was not affected at 2 and 24 h post-infection (Fig. 3A and B). Increased caspase-1 expression was observed at 2 h and continued at 24 h (Fig. 3C). However, Fig. 3D shows that the expression of ASC, a major adaptor protein involved in the inflammasome, was increased only at the early time points (1 and 2 h).
To investigate the role of NODs in the response of the host cell against M. leprae, the expression of caspase-1 in the HEK cells transfected with NOD1 or NOD2 expression plasmid was examined. Caspase-1 expression was increased in cells transfected with NOD2 (Fig. 4), not NOD1, suggesting that caspase-1-mediated IL-1β production was dependent on NOD2 signaling.
IL-1β is a pro-inflammatory cytokines that has a critical role in the prevention of intracellular pathogens, including Bacillus anthracis and Francisella (1720). Our results from the current study showed that M. leprae also induced caspase-1-mediated IL-1 secretion in RAW 274.7 cells (Fig. 1). NLRs, one of the two major classes of PRR in the innate immune system, provide a crucial interface between invading bacterial pathogens and the host immune system. Activation of NLRs by bacterial products can stimulate the NF-κB pathway, a key regulator of the pro-inflammatory response, activating genes that are involved in immune responses to stimuli. Two NLRs, NOD1 and NOD2, induce caspase-1 activation and IL-1β expression via large protein complexes named inflammasomes.
In order to more clearly define the roles of NOD1 and NOD2, we transfected HEK 293T cells with NOD1, NOD2 or both NODs. There was low response to M. leprae in NOD1-transfected cells, but a higher response in NOD2-transfected cells in caspase-1 expression (Fig. 4). We expected that HEK 293T cells transfected with both receptors (NODs) to have a significantly higher response than cells transfected with either one alone. However, there was no synergistic effect between NOD1 and NOD2 in the response to M. leprae (Fig. 4). Therefore, our results suggest that NOD2, rather than NOD1, is associated with the host response to M. leprae infection. The future study will examine the role of NODs in host infected with M. leprae using siRNA and knock-out mice.
Figures and Tables
 | Figure 1Caspase-1 activation and IL-1β production in RAW264.7 cells infected with M. leprae. Macrophages (106) were incubated with caspase-1 inhibitor (Ac-YVAD-CMK) for 1 h and then treated with LPS (100 ng/ml) and M. leprae (MOI of 10) for 18 h, and the supernatants and cell lysates were assayed for caspase-1 activity (A) and IL-1β (B), respectively. Data are representative of at least three independent experiments, each performed in triplicate. **p<0.01. |
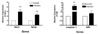 | Figure 2The expression of NOD-related genes in mouse footpads infected with M. leprae by determined by real-time PCR. Data are representative of at least three independent experiments, each performed in triplicate. *p<0.05; **p<0.01 vs. no stimulation. |
 | Figure 3The expression of NOD-related genes (A; NOD1, B; NOD2, C; caspase-1, D; ASC) in RAW 264.7 cells infected with M. leprae determined by real-time PCR. Data are representative of at least three independent experiments, each performed in triplicate. *p<0.05; **p<0.01 vs. no stimulation. |
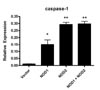 | Figure 4Caspase-1 expression in HEK cells transfected with NOD1 and NOD2. HEK cells were transfected with NOD1 or NOD2, or co-transfected with both NODs and stimulated for 24 hours with viable M. leprae at 10.0 MOI. Data are representative of at least three independent experiments, each performed in triplicate. *p<0.05; **p<0.01 vs. empty vector. |
Table I
Primers used in this study

References
1. Kang TJ, Lee SB, Chae GT. A polymorphism in the toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine. 2002; 20:56–62.

2. Kang TJ, Yeum CE, Kim BC, You EY, Chae GT. Differential production of interleukin-10 and interleukin-12 in mononuclear cells from leprosy patients with a Toll-like receptor 2 mutation. Immunology. 2004; 112:674–680.

3. Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004; 41:1099–1108.

4. Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008; 10:1–8.

5. Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007; 27:549–559.

6. Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005; 307:731–734.

7. Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003; 23:7531–7539.

8. Ferwerda G, Kramer M, de JD, Piccini A, Joosten LA, Devesaginer I, Girardin SE, Adema GJ, van der Meer JW, Kullberg BJ, Rubartelli A, Netea MG. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur J Immunol. 2008; 38:184–191.

9. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995; 267:2000–2003.

10. Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003; 278:8869–8872.

11. Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003; 278:5509–5512.
12. Coulombe F, Divangahi M, Veyrier F, de LL, Gleason JL, Yang Y, Kelliher MA, Pandey AK, Sassetti CM, Reed MB, Behr MA. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009; 206:1709–1716.

13. Ferwerda G, Girardin SE, Kullberg BJ, Le BL, de Jong DJ, Langenberg DM, van CR, Adema GJ, Ottenhoff TH, Van der Meer JW, Netea MG. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005; 1:279–285.

14. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426.
15. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004; 430:213–218.

16. Shepard CC, McRae DH. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968; 36:78–82.
17. Kang TJ, Basu S, Zhang L, Thomas KE, Vogel SN, Baillie L, Cross AS. Bacillus anthracis spores and lethal toxin induce IL-1beta via functionally distinct signaling pathways. Eur J Immunol. 2008; 38:1574–1584.

18. Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002; 70:6896–6903.

19. Kang TJ, Lee GS, Kim SK, Jin SH, Chae GT. Comparison of two mice strains, A/J and C57BL/6, in caspase-1 activity and IL-1beta secretion of macrophage to Mycobacterium leprae infection. Mediators Inflamm. 2010; 2010:708713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download